Chemistry:Campestane
From HandWiki
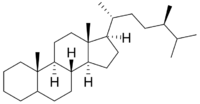
| |
| Names | |
|---|---|
| IUPAC name
5ξ-Campestane[1]
| |
| Systematic IUPAC name
(1R,3aS,3bR,5aΞ,9aS,9bS,11aR)-1-[(2R,5R)-5,6-Dimethylheptan-2-yl]-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C28H50 | |
| Molar mass | 386.708 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Campestane or 24R-methylcholestane is a tetracyclic triterpene.[2] Its derivative campesterol (campest-5-en-3β-ol) was first isolated from the rapeseed (Brassica campestris), hence the name.[3]
See also
- Cholestane
- Ergostane (24S-methylcholestane)
- Campestanol (Campestan-3β-ol)
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 1528. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ "Enantioselective catalytic approach to the C23-C28 subunit of 24α-methyl steroids". Steroids 148: 82–90. 2019. doi:10.1016/j.steroids.2019.05.003. PMID 31100291.
- ↑ Fernholz, Erhard; MacPhillamy, H. B. (1941). "Isolation of a New Phytosterol: Campesterol". Journal of the American Chemical Society 63 (4): 1155. doi:10.1021/ja01849a079.
 |

