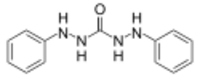
Chemistry:Carbazide
In chemistry, a carbazide is a functional group with the general formula RNH-NH(C=O)NH-NHR. They can be derived from the condensation of carbonic acid with a hydrazine. Carbohydrazide is the simplest carbazide, with another common carbazide being diphenylcarbazide, which is used as an analytical reagent.[1]
Diphenylcarbazide forms an intense blue color with chromium in the hexavalent state. It has an absorptivity coefficient of about 3400. That means very small amounts of chromium can be detected; 25 micrograms in 25 mL of solution are too dark to read on a spectral device, so concentrations well below that can be detected.
Thiocarbazide
The sulfur analog is called a thiocarbazide, of which thiocarbohydrazide is the simplest example.
Carbazone and thiocarbazone
A carbazone is a partially oxidized carbazide with the general formula R=NNH(C=O)NH-NHR. The sulfur analog is called a thiocarbazone, of which dithizone is an example.
See also
References
- ↑ Crossley, H. E. (1936). "Diphenylcarbazide. An internal indicator for use in the titration of iron with dichromate". The Analyst 61 (720): 164. doi:10.1039/AN9366100164. Bibcode: 1936Ana....61..164C.
 |