Chemistry:Carbonyl diazide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Carbonyl diazide
| |
| Other names
Carbonyl azide,
Carbonic diazide, Azido ketone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
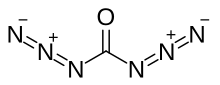
| CO(N 3) 2 | |
| Molar mass | 112.05 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carbonyl diazide is a chemical compound with the formula CO(N
3)
2. It can be prepared by way of the reaction between triphosgene and tetra-n-butylammonium azide, in a dimethyl or diethyl solution.[1]
The first synthesis of carbonyl diazide was reported in 1894, although there have been multiple alternative syntheses since then.[2]
References
- ↑ Nolan, Alex M.; Amberger, Brent K.; Esselman, Brian J.; Thimmakondu, Venkatesan S.; Stanton, John F.; Woods, R. Claude; McMahon, Robert J. (28 September 2012). "Carbonyl Diazide, OC(N3)2: Synthesis, Purification, and IR Spectrum". Inorganic Chemistry 51 (18): 9486–9851. doi:10.1021/ic301270b. PMID 22928580. https://pubs.acs.org/doi/full/10.1021/ic301270b. Retrieved 31 October 2023.
- ↑ Häring, Andreas P.; Kirsch, Stefan F. (6 November 2015). "Synthesis and Chemistry of Organic Geminal Di- and Triazides". Molecules 20 (11): 20044–20062. doi:10.3390/molecules201119675. PMID 26561796.
 |

