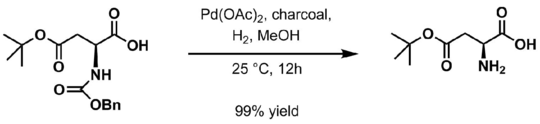Chemistry:Carboxybenzyl
Carboxybenzyl, symbol Cbz, Cbo (old symbol), or Z (in honor of its inventor Leonidas Zervas),[1] is a carbamate which is often used as an amine protecting group in organic synthesis.[2] It is commonly used in peptide synthesis where the carboxybenzyl protecting group is introduced by reacting the amine functionality with benzyl chloroformate in the presence of a weak base:
Alternatively, as in the Curtius rearrangement, it is made by the trapping of an isocyanate with benzyl alcohol.
It is used to protect amines from electrophiles. The protected amine can be deprotected by catalytic hydrogenation or treatment with HBr, yielding a terminal carbamic acid that then readily decarboxylates to yield the free amine.
The method was first used by Max Bergmann and Leonidas Zervas in 1932 for the synthesis of peptides.[3] The abbreviation Z is in honor of Zervas.
Amine protection
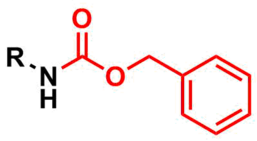
The carboxybenzyl group (Cbz) is commonly used in organic synthesis as a protecting group for amines.
Most common amine protection methods
- Benzyl chloroformate and a base, such as sodium carbonate in water at 0 °C[3]
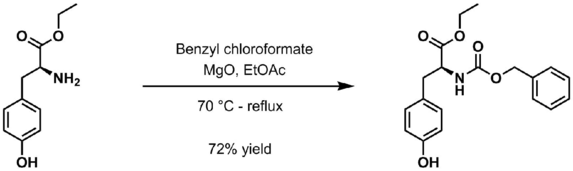
- Benzyl chloroformate and magnesium oxide in ethyl acetate at 70 °C to reflux[4]
- Benzyl chloroformate, DIPEA, acetonitrile and scandium trifluoromethanesulfonate (Sc(OTf)3)[5]
Deprotection
Hydrogenolysis in the presence of a variety of palladium-based catalysts is the usual method for deprotection. Palladium on charcoal is typical.[6]
Alternatively, strong Lewis acids have been used, provided that a trap is provided for the released benzyl carbocation.[7]
References
- ↑ Jakubke, Hans-Dieter; Sewald, Norbert (2008). Peptides from A to Z: A Concise Encyclopedia. John Wiley & Sons. ISBN 978-3-527-62117-0. https://books.google.com/books?id=doe9NwgJTAsC&pg=PA46&lpg=PA46.
- ↑ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. pp. 248, 652-654, 1484. ISBN 978-0-19-850346-0.
- ↑ 3.0 3.1 "Über ein allgemeines Verfahren der Peptid-Synthese". Berichte der deutschen chemischen Gesellschaft 65 (7): 1192–1201. 1932. doi:10.1002/cber.19320650722.
- ↑ Dymicky, M. (1989-02-01). "Preparation of Carbobenzoxy-L-Tyrosine Methyl and Ethyl Esters and of the Corresponding Carbobenzoxy Hydrazides". Organic Preparations and Procedures International 21 (1): 83–90. doi:10.1080/00304948909356350. ISSN 0030-4948.
- ↑ Aggarwal, Varinder K.; Humphries, Paul S.; Fenwick, Ashley (1999). "A Formal Asymmetric Synthesis of Anatoxin-a Using an Enantioselective Deprotonation Strategy on an Eight-Membered Ring". Angewandte Chemie International Edition 38 (13–14): 1985–1986. doi:10.1002/(SICI)1521-3773(19990712)38:13/14<1985::AID-ANIE1985>3.0.CO;2-7. http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1521-3773(19990712)38:13/14%3C1985::AID-ANIE1985%3E3.0.CO;2-7/abstract.
- ↑ Felpin, François-Xavier; Fouquet, Eric (2010-11-02). "A Useful, Reliable and Safer Protocol for Hydrogenation and the Hydrogenolysis of O-Benzyl Groups: The In Situ Preparation of an Active Pd0/C Catalyst with Well-Defined Properties" (in en). Chemistry – A European Journal 16 (41): 12440–12445. doi:10.1002/chem.201001377. ISSN 1521-3765. http://onlinelibrary.wiley.com/doi/10.1002/chem.201001377/abstract.
- ↑ Theodora W. Greene, Peter G. M. Wuts (1999). Protecting Groups in Organic Synthesis (3 ed.). J. Wiley. ISBN 0-471-16019-9.