Chemistry:CataCXium F sulf
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
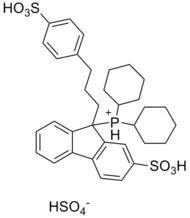
(±)-Dicyclohexyl-{9-[3-(4-sulfonylphenyl)propyl]-2-sulfonylfluoren-9-yl}phosphonium hydrogensulfate
| |
| Other names
CataCXium F sulf
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C34H43O10PS3 | |
| Molar mass | 738.87 g/mol |
| Appearance | pale yellow solid |
| Solubility | soluble in water |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
CataCXium F sulf is a water-soluble organophosphorus compound derived from fluorene. The palladium complexes of the respective phosphine[clarification needed] show an excellent activity in various palladium-catalyzed coupling reactions, including Suzuki reactions, Sonogashira couplings and Buchwald–Hartwig reactions.
References
- C.A. Fleckenstein; H. Plenio, Highly efficient Suzuki-Miyaura coupling of heterocyclic substrates through rational reaction design. Chemistry-A European Journal 2008, 14(14), 4267–4279.
- C.A. Fleckenstein; H. Plenio, Aqueous/organic cross coupling: Sustainable protocol for Sonogashira reactions of heterocycles. Green Chemistry 2008, 10, 563–570. doi:10.1039/B800154E
- C.A. Fleckenstein; H. Plenio, Efficient Suzuki-Miyaura Coupling of (Hetero)aryl Chlorides with Thiophene- and Furanboronic Acids in Aqueous n-Butanol. Journal of Organic Chemistry 2008, 73, 3236–3244.
- C.A Fleckenstein; R. Kadyrov; H. Plenio, Efficient Large-Scale Synthesis of 9-Alkylfluorenyl Phosphines for Pd-Catalyzed Cross-Coupling Reactions. Organic Process Research & Development 2008, 12, 475–479.
- C.A. Fleckenstein; H. Plenio, Aqueous cross-coupling. Highly efficient Suzuki–Miyaura coupling of N-heteroaryl halides and N-heteroarylboronic acids. Green Chemistry 2007, 9, 1287–1291. doi:10.1039/B711965H
External links
 |
