Chemistry:Cinchonain-Ib
From HandWiki
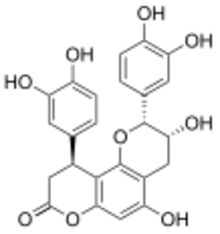
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4′′S)-4′′-(3,4-Dihydroxyphenyl)-3,3′,4′,5-tetrahydroxy-6′′H-pyrano[2′′,3′′:7,8]flavan-6′′-one
| |
| Systematic IUPAC name
(2R,3R,10S)-2,10-Bis(3,4-dihydroxyphenyl)-3,5-dihydroxy-3,4,9,10-tetrahydro-2H,8H-(benzo[1,2-b:3,4-b′]dipyran)-8-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H20O9 | |
| Molar mass | 452.415 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cinchonain-Ib is a flavonolignan found in the bark of Trichilia catigua used as catuaba.[1] A 2009 study revealed that Cinchonian-Ib derived from boiled Eriobotrya japonica leaves has a glucose-lowering effect in rats, and could possibly be used to manage diabetes mellitus in humans.[2]
References
- ↑ Beltrame, F. L.; Filho, E. R.; Barros, F. A. P.; Cortez, D. A. G.; Casset, Q. B. (2006). "A validated higher-performance liquid chromatography method for quantification of cinchonain Ib in bark and phytopharmaceuticals of Trichilia catigua used as Catuaba". Journal of Chromatography A 1119 (1–2): 257–263. doi:10.1016/j.chroma.2005.10.050. PMID 16360665.
- ↑ Qa’dan, Fadi; Verspohl, Eugen. J.; Nahrstedt, Adolf; Petereit, Frank; Matalka, Khalid Z. (15 July 2009). "Cinchonain Ib isolated from Eriobotrya japonica induces insulin secretion in vitro and in vivo" (in en). Journal of Ethnopharmacology 124 (2): 224–227. doi:10.1016/j.jep.2009.04.023. ISSN 0378-8741. PMID 19397981. https://www.sciencedirect.com/science/article/abs/pii/S0378874109002657. Retrieved 19 October 2022.
 |

