Chemistry:Cobalt oleate
From HandWiki
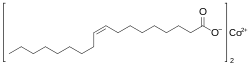
| |
| Names | |
|---|---|
| IUPAC name
Cobalt (Z)-octadec-9-enoate
| |
| Other names
Cobaltous oleate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| UNII | |
| |
| Properties | |
| C36H66CoO4 | |
| Molar mass | 621.853 g·mol−1 |
| Appearance | Purple powder |
| Solubility | Soluble in benzene, carbon tetrachloride, pyridine, chloroform, quinoline[1] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H317, H411, H412 | |
| P261, P272, P273, P280, P302+352, P321, P333+313, P362+364Script error: No such module "Preview warning".Category:GHS errors, P391, P501 | |
| Related compounds | |
Other cations
|
Sodium oleate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Cobalt oleate is an organometallic compound with the formula Co(C18H33O2)2. When cobalt oleate is added to non-polar solvents, the viscosity rapidly increases, and then continues increasing over time. This unusual viscosity effect is caused by the formation of a weak coordination complex with the solvent molecules.[1]
Preparation
Cobalt oleate can be synthesized by heating a solution of sodium oleate and cobalt(II) chloride to 70 °C.[2]
[math]\ce{ 2NaC18H33O2 + CoCl2 -> 2NaCl + Co(C18H33O2)2 }[/math]
See also
References
- ↑ Jump up to: 1.0 1.1 Funakoshi, Hideo; Matuura, Ryohei (October 1964). "Peptizing Action of Some Polar Substances on the Benzene Solution of Cobalt Oleate" (in en). Nature 204 (4954): 186. doi:10.1038/204186a0. ISSN 0028-0836. Bibcode: 1964Natur.204..186F. https://www.nature.com/articles/204186a0.
- ↑ An, Kwangjin; Lee, Nohyun; Park, Jongnam; Kim, Sung Chul; Hwang, Yosun; Park, Je-Geun; Kim, Jae-Young; Park, Jae-Hoon et al. (2006-08-01). "Synthesis, Characterization, and Self-Assembly of Pencil-Shaped CoO Nanorods" (in en). Journal of the American Chemical Society 128 (30): 9753–9760. doi:10.1021/ja0608702. ISSN 0002-7863. PMID 16866531. https://pubs.acs.org/doi/10.1021/ja0608702.
 |

