Chemistry:Corey-Seebach reaction
| Corey-Seebach reaction | |
|---|---|
| Named after | Elias James Corey Dieter Seebach |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | corey-seebach-reaction |
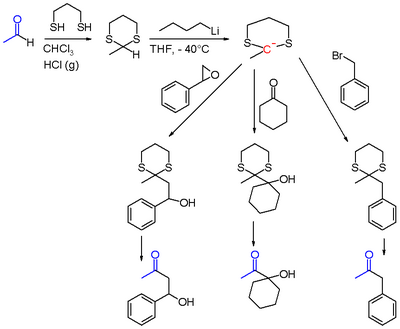
The Corey-Seebach reaction, or Seebach Umpolung is a name reaction of organic chemistry that allows for acylation by converting aldehydes into lithiated 1,3-dithianes. The lithiated 1,3-dithianes serves as an acyl anion equivalent, undergoing alkylation with electrophiles.[1][2][3][4] The reaction is of pedagogical value, but it is cumbersome, so it is not widely used.
Implementation
The aldehyde is first converted into a dithiane, usually with 1,3-propanedithiol. The resulting 1,3-dithiane is then lithiated with the use of butyllithium. The 2-lithio-1,3-dithiane reacts with electrophiles to give a 2-alkyl-1,3-dithiane.[3]
Finally, the 2-alkyl-1,3-dithiane can be converted to a carbonyl by hydrolysis, usually with the use of mercury(II) oxide. Alternatively the 2-alkyl-1,3-dithiane can be reduced to an alkane.[5]
Scope
The lithiated 1,3-dithiane can react with alkyl halides, epoxides,[6][7] ketones, acyl halides, and iminium salts, which after hydrolysis of dithioacetals can yield ketones, β-hydroxyketones, α-hydroxyketones, 1,2-diketones[1] and α-aminoketones. Notably, α-hydroxyketones and 1,2-diketones can not be generated through typical reactions of aldehydes such as the aldol reaction.[8] Other possible electrophiles include aldehydes,[9] amides, and esters.[8][10]
The reaction between lithiated 1,3-dithianes and arenesulfonates offers a similar path to that. of alkyl halides, being able to form dithioacetals which can be converted to ketones.[11]
References
- ↑ 1.0 1.1 Corey, Elias James; Seebach, Dieter (1 December 1965). "Synthesis of 1,n-Dicarbonyl Derivates Using Carbanions from 1,3-Dithianes". Angewandte Chemie International Edition 4 (12): 1077-1078. doi:10.1002/anie.196510771.
- ↑ Corey, Elias James; Seebach, Dieter (1 December 1966). "Phenylthiomethyllithium and Bis(phenylthio)methyllithium". The Journal of Organic Chemistry 31 (12): 4097–4099. doi:10.1021/jo01350a052.
- ↑ 3.0 3.1 Seebach, Dieter; Corey, Elias James (1 January 1975). "Generation and synthetic applications of 2-lithio-1,3-dithianes". The Journal of Organic Chemistry 40 (2): 231–237. doi:10.1021/jo00890a018.
- ↑ Smith, Amos B.; Adams, Christopher M. (6 May 2004). "Evolution of Dithiane-Based Strategies for the Construction of Architecturally Complex Natural Products". Accounts of Chemical Research 37 (6): 365–377. doi:10.1021/ar030245r.
- ↑ Yus, Miguel; Nájera, Carmen; Foubelo, Francisco (11 August 2003). "The role of 1,3-dithianes in natural product synthesis". Tetrahedron 59 (13): 6147-6212. doi:10.1016/S0040-4020(03)00955-4.
- ↑ Williams, David R.; Sit, Sing Yuen (1 May 1984). "Total synthesis of (+)-phyllanthocin". Journal of the American Chemical Society 106 (10): 2949–2954. doi:10.1021/ja00322a035.
- ↑ Smith, Amos B.; Xian, Ming (11 January 2006). "Anion Relay Chemistry: An Effective Tactic for Diversity Oriented Synthesis". Journal of the American Chemical Society 128 (1): 66-67. doi:10.1021/ja057059w.
- ↑ 8.0 8.1 Page, Philip C. Bulman; van Niel, Monique B.; Pordger, Jeremy C. (29 November 1988). "Synthetic uses of the 1,3-dithiane grouping from 1977 to 1988". Tetrahedron 45 (24): 7643-7677. doi:10.1016/S0040-4020(01)85784-7.
- ↑ Ley, Steven V.; Abad-Somovilla, Antonio; Anderson, James C.; Ayats, Carles; Bänteli, Rolf; Beckmann, Edith; Boyer, Alistair; Brasca, Maria G. et al. (1 January 2018). "The synthesis of azadirachtin: a potent insect antifeedant". Chemistry 14 (34): 10683-10704. doi:10.1002/chem.200801103.
- ↑ Chen, Yue-Lei; Leguijt, Robin; Redlich, Hartmut; Fröhlich, Roland (30 April 2006). "Trimethylene Dithioacetals of Carbohydrates, Part 6: C-C Coupling Reactions of Dilithiated N-Acetyl-D-glucosamine Trimethylene Dithioacetal Derivatives". Synthesis (24): 4212-4218. doi:10.1055/s-2006-950359.
- ↑ Seebach, Dieter; Wilka, Eva-Maria (1976). "Alkylation of 2-Lithio-1,3-dithianes with Arenesulfonates of Primary Alcohols". Synthesis (7): 476-477. doi:10.1055/s-1976-24092.