Chemistry:Dithiane
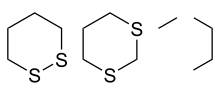 1,2-dithiane (left), 1,3-dithiane and 1,4-dithiane (right)
| |
| Names | |
|---|---|
| Other names
Dithiacyclohexanes
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4H8S2 | |
| Molar mass | 120.23 g·mol−1 |
| Melting point | 32.5 °C (90.5 °F; 305.6 K) other isomers: 54 (1,3), 112.3 (1,4) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene bridges (-CH2- units) are replaced by sulfur. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane. They are all colorless solids.
1,2-Dithianes
1,2-Dithiane is an organosulfur compound with the formula S
2C
4H
8. It is one of three isomers of the formula (CH
2)
4S
2. The 1,2-isomer, a disulfide, arises by the oxidation of 1,4-butanedithiol.
1,3-Dithianes
1,3-Dithiane is an organosulfur compound with the formula CH
2S
2C
3H
6. It is one of three isomers of the formula (CH
2)
4S
2. The 1,3-isomer arises by the reaction of 1,3-propanedithiol with formaldehyde.[1]
1,3-Dithianes are sometimes used as protecting group of carbonyl-containing compounds. They form by treatment of the carbonyl compound with 1,3-propanedithiol under conditions that remove water from the system. The protecting group can be removed with mercuric reagents, a process that exploits the high affinity of Hg(II) for thiolates. 1,3-Dithianes are more importantly employed in umpolung reactions, such as the Corey–Seebach reaction:[2]
1,4-Dithianes
1,4-Dithiane is an organosulfur compound with the formula (SC
2H
4)
2. It is one of three isomers of the formula (CH
2)
4S
2. The 1,4-isomer, a bisthioether, arises by the alkylation of 1,2-ethanedithiol with 1,2-dibromoethane. It has no applications but traces occurs as a product of degradations, e.g., cooking[3] coal pyrolysis.[4]
References
- ↑ Corey, E. J.; Seebach, D (1970). "1,3-Dithiane". Organic Syntheses 50: 72. doi:10.15227/orgsyn.050.0072.
- ↑ Wuts, P. G. M.; Greene, T.W. (2006). Greene's Protective Groups in Organic Synthesis. NY: J. Wiley. doi:10.1002/0470053488. ISBN 9780470053485.
- ↑ Garbusov, V.; Rehfeld, G.; Wölm, G.; Golovnja, R. V.; Rothe, M. (1976). "Volatile sulfur compounds contributing to meat flavour. Part. I. Components identified in boiled meat". Molecular Nutrition & Food Research 20 (3): 235–241. doi:10.1002/food.19760200302.
- ↑ Calkins, William H. (1987). "Investigation of organic sulfur-containing structures in coal by flash pyrolysis experiments". Energy Fuels 1 (1): 59–64. doi:10.1021/ef00001a011.
External links
 |



