Chemistry:Corrinoid
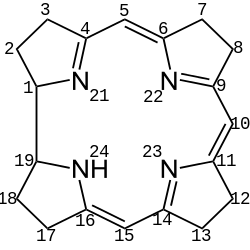
Corrinoids are a group of compounds based on the skeleton of corrin, a cyclic system containing four pyrrole rings similar to porphyrins.[2] These include compounds based on octadehydrocorrin, which has the trivial name corrole.[3]
The cobalamins (vitamin B12) are the best known members of the group. Other prominent examples include cobyrinic acid and its hexaamide cobyric acid; cobinic acid and its hexaamide cobinamide; cobamic acid and cobamide.
Compounds containing the "Cob-" prefix (not corrin) are cobalt derivatives, and may include an oxidation state, as in "Cob(II)alamin". When cobalt is replaced by another metal or hydrogen, the name changes accordingly, as in ferrobamic acid or hydrogenobamic acid.
Reactions with cyanide
A solution of aquacyano-corrinoids, such as cobalamin or cobinamide, reacts with free cyanide in an aqueous sample. The binding of cyanide to the corrinoid cobalt center leads to a color change from orange to violet.[4] Quantification of the cyanide content is feasible by UV-vis spectroscopy.[5][6] Absorption of the corrinoid on a solid phase,[7] allows detection of cyanide even in colored samples, rendering this method appropriate for the analysis of cyanide in water, wastewater, blood, and food.[8][9] Furthermore, this technology is non-toxic and considerably less prone to interference than the pyridine-barbituric acid colorimetry method.
References
- ↑ Dorothy Crowfoot Hodgkin (1965-11-16). "The Structure of the Corrin Nucleus from X-ray Analysis". Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences 288 (1414): 294–305. doi:10.1098/rspa.1965.0219. Bibcode: 1965RSPSA.288..294H.
- ↑ Cracan, Valentin; Banerjee, Ruma (2013). "Chapter 10 Cobalt and Corrinoid Transport and Biochemistry". in Banci, Lucia. Metallomics and the Cell. Metal Ions in Life Sciences. 12. Springer. pp. 333–374. doi:10.1007/978-94-007-5561-1_10. ISBN 978-94-007-5560-4. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ↑ "The Nomenclature of Corrinoids: Recommendations 1975". IUPAC-IUB Commission on Biochemical Nomenclature (CBN). 1975. http://www.chem.qmul.ac.uk/iupac/misc/B12.html.
- ↑ Pratt, J.M. (1972). Inorganic Chemistry of Vitamin B12. Academic Press. p. 44.
- ↑ Zelder, F.H. (2008). "Specific Colorimetric Detection of Cyanide Triggered by a Conformational Switch in Vitamin B12". Inorganic Chemistry 47 (4): 1264–1266. doi:10.1021/ic702368b. PMID 18205304.
- ↑ Mannel-Croise, Zelder (2009). "Side chains of cobalt corrinoids control the sensitivity and selectivity in the colorimetric detection of cyanide". Inorganic Chemistry 48 (4): 1272–1274. doi:10.1021/ic900053h. PMID 19161297.
- ↑ Mannel-Croise, Zelder (2012). "Complex samples cyanide detection with immobilized corrinoids". ACS Applied Materials & Interfaces 4 (2): 725–729. doi:10.1021/am201357u. PMID 22211318.
- ↑ Tivana, Da Cruz Francisco, Zelder, Bergenståhl, Dejmek (2014). "Straightforward rapid spectrophotometric quantification of total cyanogenic glycosides in fresh and processed cassava products". Food Chemistry 158: 20–27. doi:10.1016/j.foodchem.2014.02.066. PMID 24731309. http://www.zora.uzh.ch/id/eprint/106811/1/1-s2.0-S0308814614002519-main.pdf.
- ↑ Mannel-Croise, Zelder (2012). "Rapid visual detection of blood cyanide". Analytical Methods 4 (9): 2632–2634. doi:10.1039/c2ay25595b. http://www.zora.uzh.ch/id/eprint/75059/1/c2ay25595b.pdf.
External links
- Corrinoids at the US National Library of Medicine Medical Subject Headings (MeSH)
- "The Nomenclature of Corrinoids" at chem.qmul.ac.uk
- Goldbook
 |
