Chemistry:Cyclododecane
Cyclododecane is an organic compound with the chemical formula (CH2)12.[1] It is a waxy white solid at room temperature,[2]: 17 and is soluble in nonpolar organic solvents.
It is an intermediate of Nylon 12, polyesters, and synthetic lubricating oils.[1]: 8.1 It is also used as a temporary binder to stabilise fragile objects or to seal water-sensitive parts; it slowly sublimates over days or weeks without leaving any residue.[2]: 17
Synthesis
Cyclododecane is produced industrially through catalytic trimerisation of butadiene to cyclododecatriene, followed by hydrogenation.[3]
Uses
It is a precursor to laurolactam, a precursor to the polymer Nylon 12.[4]
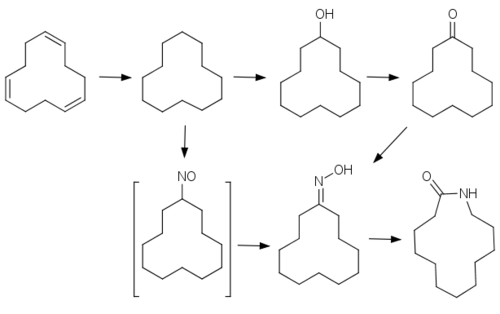
Cyclododecane is also an intermediate in production of flame retardants, detergents, and other chemicals.
Cyclododecane is also used as a volatile binding medium, a temporary binder for sealing and conservation of friable and structurally weak materials, e.g. during excavation and transport of archaeological objects and in art restoration, e.g. to protect water-sensitive parts during cleaning.[2] Due to its relatively slow evaporation in comparison with other volatile binding mediums the layer can last for several weeks. Very pure material has to be used so it does not leave any residue. Cyclododecane can be applied in molten state or dissolved in a nonpolar organic solvent. Other volatile binding mediums in use are camphene, tricyclene and with some limits menthol.
Environmental considerations
Cyclododecane is persistent in the environment, as it does not biodegrade easily. Cyclododecane is lipophilic, usually present in the environment as adsorbed on the surface of soil particles. It has the potential to bioaccumulate. Cyclododecane may cause long lasting harmful effects to aquatic life.[5]
Conformation
Cyclododecane has low ring strain. It adopts a chiral [3333] conformation with square (D4) symmetry.[6][7][8]: Fig. 70 [9]: 24 While highly stable, this conformation is not derivable from a diamond lattice,[8] unlike the lowest-energy conformations of cyclohexane, cyclotetradecane, and cyclohexadecane.[7] Monosubstituted cyclododecanes also typically adopt the [3333] conformation,[10]: 5916 though more highly substituted cyclododecanes may adopt alternative conformations, such as [4332].[11]: 10583
References
- ↑ 1.0 1.1 Template:Cite PubChem
- ↑ 2.0 2.1 2.2 Rowe, Sophie; Rozeik, Christina (2008). "The uses of cyclododecane in conservation". Studies in Conservation 53: 17–31. doi:10.1179/sic.2008.53.Supplement-2.17.
- ↑ Arpe, Hans-Jürgen (12 March 2007). Industrielle Organische Chemie. John Wiley & Sons. p. 291. ISBN 978-3-527-31540-6.
- ↑ Schiffer, T.; Oenbrink, G. (2009). "Cyclododecanol, Cyclododecanone, and Laurolactam". Ullman's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a08_201.pub2. ISBN 978-3527306732.
- ↑ "Cyclododecane". European Chemicals Agency. https://echa.europa.eu/brief-profile/-/briefprofile/100.005.486.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedAtavin1989 - ↑ 7.0 7.1 Wilen, Samuel H.; Eliel, Ernest Ludwig; Mander, Lewis N. (1994). Stereochemistry of organic compounds. New York: Wiley. p. 769. ISBN 9780471016700. https://archive.org/details/stereochemistryo0000elie_a9t3/page/768/mode/2up?q=3333.
- ↑ 8.0 8.1 Dragojlovic, Veljko (September 2015). "Conformational analysis of cycloalkanes". ChemTexts 1 (3). doi:10.1007/s40828-015-0014-0. https://link.springer.com/article/10.1007/s40828-015-0014-0#Fig70.
- ↑ "Condis crystals of cyclic alkanes, silanes and related compounds". Conformational Motion and Disorder in Low and High Molecular Mass Crystals. Berlin, Heidelberg: Springer-Verlag Springer e-books. 1988. pp. 26–44. doi:10.1007/BFb0008610. ISBN 978-3-540-38867-8.
- ↑ Khorasani, Sanaz; Fernandes, Manuel A.; Perry, Christopher B. (5 December 2012). "Do 12-Membered Cycloalkane Rings Only Exist As One Conformation in the Solid-State? A Detailed Solid-State Analysis Involving Polymorphs of N,N'-Biscyclododecyl Pyromellitic Diimide". Crystal Growth & Design 12 (12): 5908–5916. doi:10.1021/cg300765b.
- ↑ Skibinski, Maciej; Wang, Yi; Slawin, Alexandra M. Z.; Lebl, Tomas; Kirsch, Peer; O'Hagan, David (4 November 2011). "Alicyclic Ring Structure: Conformational Influence of the CF2 Group in Cyclododecanes". Angewandte Chemie International Edition 50 (45): 10581–10584. doi:10.1002/anie.201105060.
External links
 |
