Chemistry:DMTMM
DMTMM (4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride) is an organic triazine derivative commonly used for activation of carboxylic acids, particularly for amide synthesis. Amide coupling is one of the most common reactions in organic chemistry and DMTMM is one reagent used for that reaction. The mechanism of DMTMM coupling is similar to other common amide coupling reactions involving activated carboxylic acids.[1] Its precursor, 2-chloro-4,6,-dimethoxy-1,3,5-triazine (CDMT), has also been used for amide coupling. DMTMM has also been used to synthesize other carboxylic functional groups such as esters and anhydrides. DMTMM is usually used in the chloride form but the tetrafluoroborate salt is also commercially available.[2]
Synthesis
DMTMM is prepared by reaction of 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) with N-methylmorpholine (NMM). It was first reported in 1999.[3] CDMT spontaneously reacts with NMM to form the quaternary ammonium chloride salt of DMTMM. DMTMM should be stored at -20 °C and kept dry.
Reactions
Amides
Amides can be readily prepared from the corresponding carboxylic acid and amine using DMTMM coupling. DMTMM has been shown to be preferable to other coupling agents in several cases, such as for sterically hindered amines[4] and for ligation of polysaccharides such as hyaluronic acid.[5][6]
Other carboxylic derivatives
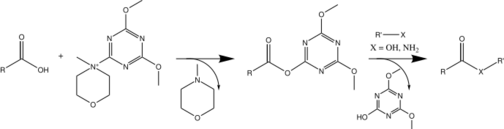
Despite primarily being used for amide synthesis, DMTMM can also be used to make esters from the corresponding alcohol and carboxylic acid.[3][7] DMTMM has also been applied to anhydride synthesis.[7] The synthesis of each carboxylic derivative is similar, relying on the activation of the starting carboxylic acid followed by nucleophilic attack by another molecule.
Coupling mechanism
DMTMM uses a typical mechanism to form carboxylic acid derivatives.[1] First, the carboxylic acid reacts with DMTMM to form the active ester, releasing a molecule of N-methylmorpholinium (NMM). The resulting ester is highly reactive and can undergo a nucleophilic attack by an amine, an alcohol, or another nucleophile.[3] A molecule of 4,6,-dimethoxy-1,3,5-triazin-2-ol is released and the corresponding carboxylic derivative is formed.
Safety
DMTMM may be toxic if ingested.[8] In vivo dermal sensitization studies according to OECD 429[9] confirmed DMTMM is a moderate skin sensitizer, showing a response at 0.9 wt% in the Local Lymph Node Assay (LLNA) placing it in Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Dermal Sensitization Category 1A.[10] Protective gloves, lab coats, and eye protection should be employed to reduce exposure while using DMTMM.
References
- ↑ 1.0 1.1 Valeur, Eric; Bradley, Mark (2009-01-26). "Amide bond formation: beyond the myth of coupling reagents" (in en). Chemical Society Reviews 38 (2): 606–631. doi:10.1039/B701677H. ISSN 1460-4744. PMID 19169468.
- ↑ "4-
(4,6- Dimethoxy- 1,3,5- triazin- 2- yl) - 4- methylmorpholinium chloride 74104". http://www.sigmaaldrich.com/catalog/product/aldrich/74104?lang=en®ion=US. - ↑ 3.0 3.1 3.2 Kunishima, Munetaka; Kawachi, Chiho; Monta, Jun; Terao, Keiji; Iwasaki, Fumiaki; Tani, Shohei (1999). "4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride: an efficient condensing agent leading to the formation of amides and esters". Tetrahedron 55 (46): 13159–13170. doi:10.1016/s0040-4020(99)00809-1.
- ↑ Shieh, Wen-Chung; Chen, Zhuoliang; Xue, Song; McKenna, Joe; Wang, Run-Ming; Prasad, Kapa; Repič, Oljan (2008). "Synthesis of sterically-hindered peptidomimetics using 4-(4,6-dimethoxy-1,3,5-triazine-2-yl)-4-methyl-morpholinium chloride". Tetrahedron Letters 49 (37): 5359–5362. doi:10.1016/j.tetlet.2008.06.119.
- ↑ D’Este, Matteo; Eglin, David; Alini, Mauro (2014). "A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to Hyaluronan in water". Carbohydrate Polymers 108: 239–246. doi:10.1016/j.carbpol.2014.02.070. PMID 24751270.
- ↑ FARKAS, P; BYSTRICKY, S (2007). "Efficient activation of carboxyl polysaccharides for the preparation of conjugates". Carbohydrate Polymers 68 (1): 187–190. doi:10.1016/j.carbpol.2006.07.013.
- ↑ 7.0 7.1 Armitt, David J. (2001). "Focus: 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium Chloride (DMTMM)" (in en). Australian Journal of Chemistry 54 (7): 469. doi:10.1071/ch01157. ISSN 1445-0038.
- ↑ "MSDS - 74104". http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=74104&brand=ALDRICH&PageToGoToURL=http://www.sigmaaldrich.com/catalog/product/aldrich/74104?lang=en.
- ↑ "Test No. 429: Skin Sensitisation" (in en). 2010-07-22. https://www.oecd.org/en/publications/test-no-429-skin-sensitisation_9789264071100-en.html.
- ↑ Graham, Jessica C.; Trejo-Martin, Alejandra; Chilton, Martyn L.; Kostal, Jakub; Bercu, Joel; Beutner, Gregory L.; Bruen, Uma S.; Dolan, David G. et al. (2022-06-20). "An Evaluation of the Occupational Health Hazards of Peptide Couplers". Chemical Research in Toxicology 35 (6): 1011–1022. doi:10.1021/acs.chemrestox.2c00031. ISSN 0893-228X. PMC 9214767. https://doi.org/10.1021/acs.chemrestox.2c00031.
 |