Chemistry:Diazinane
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| |
| |
| Properties | |
| C4H10N2 | |
| Molar mass | 86.138 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
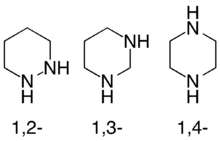
Diazinanes or hexahydrodiazines are a class of nitrogen-containing heterocycles consisting of a saturated four-carbon, two-nitrogen ring. They exist in three isomeric forms depending on the relative position of the two nitrogen atoms, with 1,4-diazinanes being common.
Structure
The diazinanes have six-membered cyclohexane-like ring but with two carbons replaced by nitrogens. The three isomers of triazinane are distinguished by the positions of their nitrogen atoms, and are referred to as 1,2-diazinane, 1,3-diazinane, and 1,4-diazinane (more commonly called piperazine).
References
See also
- 6-membered saturated rings with one nitrogen atom: Piperidine
- 6-membered saturated rings with two nitrogen atoms: Diazinane
- Piperazine
- Hexahydropyrimidine
- Hexahydropyridazine
- 6-membered aromatic rings with two nitrogen atoms: Diazine
- 6-membered saturated rings with three nitrogen atoms Triazinane
 |

