Chemistry:Diberal
| |
| |
| Names | |
|---|---|
| IUPAC name
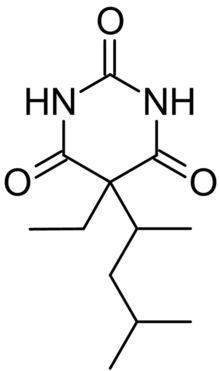
5-ethyl-5-(4-methylpentan-2-yl)-1,3-diazinane-2,4,6-trione
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H20N2O3 | |
| Molar mass | 240.30 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diberal, also known as 5-(1,3-Dimethylbutyl)-5-ethylbarbituric acid or DMBB, is an atypical barbiturate. This compound can be either convulsant or anticonvulsant depending on which enantiomer is used.
Pharmacology
Diberal, unlike most barbiturates, can have convulsant actions. This is uncommon, as barbiturates typically enhance the function of GABA as allorestic modulators and agonists (at higher doses)[2], therefore having anticonvulsant properties. Depending on which isomer is used, it can have either convulsant or anticonvulsant actions.
The different pharmacological profile between isomers is thought to be due to the differences in the formation of hydrogen bonds at the binding sites.[3]
(+) Isomer
(+)-DMBB is the atypical enantiomer of diberal. It is atypical in the means that it has convulsant action, unlike most barbiturate drugs.[4]
(-) Isomer
Unlike (+)-DMBB, the (-) isomer is similar to most barbiturates by having anticonvulsant action.
It has been found that administration of (-)-DMBB reverses the convulsant actions of (+)-DMBB.[3]
(-)-DMBB is slightly more potent than pentobarbital in its depressant properties.[5]
References
- ↑ "Diberal". https://pubchem.ncbi.nlm.nih.gov/compound/18079.
- ↑ Suddock, J. T.; Kent, K. J.; Cain, M. D. (2024). "Barbiturate Toxicity". StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK499875/.
- ↑ 3.0 3.1 "Mechanism of Action of Barbiturates". https://flexiblelearning.auckland.ac.nz/medsci303/6/files/review_article_on_barbiturates.pdf.
- ↑ Desai, Rooma; Savechenkov, Pavel Y.; Zolkowska, Dorota; Ge, Ri Le; Rogawski, Michael A.; Bruzik, Karol S.; Forman, Stuart A.; Raines, Douglas E. et al. (2015). "Contrasting actions of a convulsant barbiturate and its anticonvulsant enantiomer on the α1β3γ2L GABAA receptor account for their in vivo effects". The Journal of Physiology 593 (22): 4943–4961. doi:10.1113/JP270971. PMID 26378885.
- ↑ https://jpet.aspetjournals.org/content/175/3/692
