Chemistry:Diethofencarb
From HandWiki
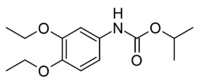
| |
| Names | |
|---|---|
| Preferred IUPAC name
propan-2-yl N-(3,4-diethoxyphenyl)carbamate | |
| Other names
Diethofencarb
| |
| Identifiers | |
3D model (JSmol)
|
|
| 8393454 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H21N1O4 | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| P264+265Script error: No such module "Preview warning".Category:GHS errors, P280, P305+351+338, P337+317Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Diethofencarb is a carbamate fungicide which is used to control Botrytis infections on a variety of fruit and vegetable crops.[2][3]
References
- ↑ "Diethofencarb" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/91742#section=Safety-and-Hazards.
- ↑ "Mechanisms of resistance to fungicides in field strains of Botrytis cinerea". Pest Management Science 58 (9): 876–888. September 2002. doi:10.1002/ps.566. PMID 12233177.
- ↑ "Shift of Sensitivity in Botrytis cinerea to Benzimidazole Fungicides in Strawberry Greenhouse Ascribing to the Rising-lowering of E198A Subpopulation and its Visual, On-site Monitoring by Loop-mediated Isothermal Amplification". Scientific Reports 9 (1): 11644. August 2019. doi:10.1038/s41598-019-48264-4. PMID 31406191. Bibcode: 2019NatSR...911644L.
 |

