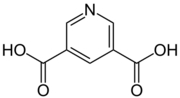
Chemistry:Dinicotinic acid
From HandWiki
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Pyridine-3,5-dicarboxylic acid | |
| Other names
3,5-Pyridinedicarboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 131640 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 279307 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H5NO4 | |
| Molar mass | 167.120 g·mol−1 |
| Structure[1] | |
| monoclinic | |
| P21/c, No. 14 | |
a = 9.702 Å, b = 11.153 Å, c = 6.587 Å α = 90°, β = 107.80°, γ = 90°
| |
Formula units (Z)
|
4 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Dinicotinic acid (pyridine-3,5-dicarboxylic acid) is a heterocyclic organic compound, more precisely a heteroaromatic. It is one of many pyridinedicarboxylic acids and consists of a pyridine ring carrying to carboxy groups in the 3- and 5-positions.
Preparation and properties
Dinicotinic acid can be formed by heating pyridine-2,3,5,6-tetracarboxylic acid or carbodinicotinic acid (pyridine-2,3,5-tricarboxylic acid).[2][3]
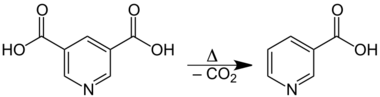
The acid is sparingly soluble in water and ether. Its melting point of 323 °C is the highest among pyridinedicarboxylic acids. Upon heating, it decarboxylates and decomposes to nicotinic acid:[4]
References
- ↑ Structure reports for 1973 : organic section. Organic compounds. J. Trotter. Dordrecht: Springer. 1975. pp. 174. ISBN 978-94-017-3121-8. OCLC 859588799. https://books.google.com/books?id=3H7vCAAAQBAJ&pg=PA174.
- ↑ Meyer, Hans; Tropsch, Hans (1914). "Über Dinicotinsäure und deren Abbau zu ββ′-Diaminopyridin und über das αα′-Diaminopyridin" (in de). Monatshefte für Chemie 35 (2): 207–217. doi:10.1007/BF01518124. ISSN 0026-9247. https://zenodo.org/record/1814560.
- ↑ Wolffenstein, Richard (1922) (in de). Die Pflanzenalkaloide (Dritte, verbesserte und vermehrte Auflage ed.). Berlin, Heidelberg. pp. 67. ISBN 978-3-642-92449-1. OCLC 913710178. https://www.worldcat.org/oclc/913710178.
- ↑ Alam, Mahbub; Khan, M. Hafeez (1980). "Preparation of some nicotinic acid derivatives". Philippine Journal of Science 109 (1–2): 19–21.
See also
- Dipicolinic acid, an isomeric dicarboxylic acid
 |