Chemistry:Enterodiol
From HandWiki
Short description: Lignan formed by the action of intestinal bacteria on lignan precursors found in plants.[1]
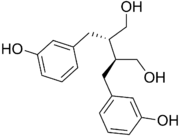
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,3R)-2,3-Bis[(3-hydroxyphenyl)methyl]butane-1,4-diol | |
| Other names
(−)-Enterodiol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H22O4 | |
| Molar mass | 302.370 g·mol−1 |
| Appearance | colorless |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Enterodiol is an organic compound with the formula [HOC6H4CH2CH(CH2OH)]2.
It is formed by the action of intestinal bacteria on lignan precursors. As such it is sometimes classified as a enterolignan or mammalian lignan.[1][2] Elevated levels of enterodiol in urine are attributed consumption of tea and other lignan-rich foods.[3]
References
- ↑ Adlercreutz, Herman (2007). "Lignans and Human Health". Critical Reviews in Clinical Laboratory Sciences 44 (5–6): 483–525. doi:10.1080/10408360701612942. PMID 17943494.
- ↑ Lampe JW (2003). "Isoflavonoid and lignan phytoestrogens as dietary biomarkers". J Nutr 133 (Suppl 3): 956S–964S. doi:10.1093/jn/133.3.956S. PMID 12612182.
- ↑ Adlercreutz, H.; Honjo, H.; Higashi, A.; Fotsis, T.; Hämäläinen, E.; Hasegawa, T.; Okada, H. (1991). "Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese Diet". The American Journal of Clinical Nutrition 54 (6): 1093–1100. doi:10.1093/ajcn/54.6.1093. PMID 1659780.
 |
