Chemistry:Epicocconone
| |
| Names | |
|---|---|
| Preferred IUPAC name
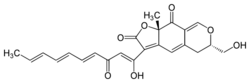
(6S,9aS)-6-(Hydroxymethyl)-3-[(1Z,4E,6E,8E)-1-hydroxy-3-oxodeca-1,4,6,8-tetraen-1-yl]-9a-methyl-5,6-dihydro-2H-furo[3,2-g][2]benzopyran-2,9(9aH)-dione | |
| Other names
Deep Purple
Lava Purple | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H22O7 | |
| Molar mass | 410.422 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Epicocconone is a long Stokes' shift fluorogenic natural product found in the fungus Epicoccum nigrum.[1] Though weakly fluorescent in water (green emission, 520 nm) it reacts covalently yet reversibly with primary amines such as those in proteins to yield a product with a strong orange-red emission (610 nm).[2] Epicoconone is notable because it the first covalent/reversible/turn-on fluorophore to be discovered and is a natural product with a new fluorescent scaffold. It is also cell membrane permeable, unlike many other fluorophores, and subsequently can be used in in vivo (live cell) applications.[2] Additionally, this dye can be used as a sensitive total protein stain for 1D and 2D electrophoresis,[3] quantitative determination of protein concentration,[4] making it a powerful loading control for Western blots.[5]
Synthetic variant
In addition to the natural variant from the fungus, there are several synthetic analogs.[6] With respect to protein staining properties there are few differences between natural and synthetic analogs.
Reaction
References
- ↑ Bell, P. J. L.; Karuso, P. (2003). "Epicocconone, A Novel Fluorescent Compound from the Fungus Epicoccum nigrum". Journal of the American Chemical Society 125 (31): 9304–9305. doi:10.1021/ja035496+. PMID 12889954.
- ↑ 2.0 2.1 Choi, H. -Y.; Veal, D. A.; Karuso, P. (2005). "Epicocconone, A New Cell-Permeable Long Stokes' Shift Fluorescent Stain for Live Cell Imaging and Multiplexing". Journal of Fluorescence 16 (4): 475–482. doi:10.1007/s10895-005-0010-7. PMID 16328703.
- ↑ MacKintosh, J. A.; Choi, H. Y.; Bae, S. H.; Veal, D. A.; Bell, P. J.; Ferrari, B. C.; Van Dyk, D. D.; Verrills, N. M. et al. (2003). "A fluorescent natural product for ultra sensitive detection of proteins in one-dimensional and two-dimensional gel electrophoresis". Proteomics 3 (12): 2273–88. doi:10.1002/pmic.200300578. PMID 14673778.
- ↑ MacKintosh, J. A.; Veal, D. A.; Karuso, P. (2005). "Fluoroprofile, a fluorescence-based assay for rapid and sensitive quantitation of proteins in solution". Proteomics 5 (18): 4673–4677. doi:10.1002/pmic.200500095. PMID 16267819.
- ↑ Moritz, C. P.; Marz, S. X.; Reiss, R; Schulenborg, T; Friauf, E (2014). "Epicocconone staining: A powerful loading control for Western blots". Proteomics 14 (2–3): 162–8. doi:10.1002/pmic.201300089. PMID 24339236.
- ↑ Peixoto, P. A.; Boulangé, A; Ball, M; Naudin, B; Alle, T; Cosette, P; Karuso, P; Franck, X (2014). "Design and synthesis of epicocconone analogues with improved fluorescence properties". Journal of the American Chemical Society 136 (43): 15248–56. doi:10.1021/ja506914p. PMID 25271695.
- ↑ Coghlan, Daniel R.; Mackintosh, James A.; Karuso, Peter (2005-05-17). "Mechanism of Reversible Fluorescent Staining of Protein with Epicocconone". Organic Letters (American Chemical Society (ACS)) 7 (12): 2401–2404. doi:10.1021/ol050665b. ISSN 1523-7060.
 |

