Chemistry:Ethephon
Ethephon is an organophosphorus compound with the formula (HO)
2P(O)CH
2CH
2Cl. It is a colorless solid that is used as plant growth regulator.[1]
Mechanism of action
Upon metabolism by the plant, it is converted into ethylene, a potent regulator of plant growth and ripeness. When applied in a plant that is in a vegetative stage, ethylene usually acts by hindering vegetative growth and inducing the start of the flowering stage. If applied in a later stage, it can accelerate the ripening of some fruits.[2]
It is also a butyrylcholinesterase inhibitor.[3]
Uses in various crops
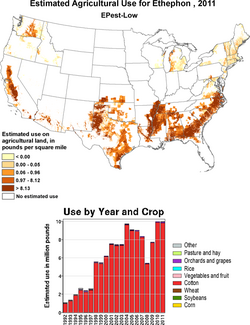
Ethephon is often used on wheat, coffee, tobacco, cotton, rice, sapote,[4]and sugar cane[5] in order to help the plant's fruit reach ripeness more quickly.
Cotton is the most important single crop use for ethephon. It initiates fruiting over a period of several weeks, promotes early concentrated boll opening, and enhances defoliation to facilitate and improve the efficiency of scheduled harvesting. Harvested cotton quality is improved.[6][7]
Ethephon is also widely used by pineapple growers to initiate the fruit's reproductive development (forcing). Ethephon is also sprayed on mature-green pineapple fruits to degreen them to meet produce marketing requirements. There can be some detrimental effect on fruit quality.[citation needed]
It is sometimes used by cannabis growers to induce flowering, abort seed formation, increase the quality of the resins, induce the appearance of female flowers in male plants, and to suppress the development of male flowers in hermaphrodite plants.[8]
The toxicity of ethephon is very low,[9] and any ethephon used on the plant is converted very quickly to ethylene.[10]
The use of this chemical is allowed in the European Union.
References
- ↑ "R.E.D. Facts Ethephon". Environmental Protection Agency. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-099801_1-Apr-95.pdf.
- ↑ Zhang, Wei; Hu, Wenli; Wen, Chi-Kuang (2010). "Ethylene preparation and its application to physiological experiments". Plant Signaling & Behavior 5 (4): 453–457. doi:10.4161/psb.5.4.10875. PMID 20118671. Bibcode: 2010PlSiB...5..453Z.
- ↑ Zhang, Nanjing; Casida, John E (2002). "Novel Irreversible Butyrylcholinesterase Inhibitors: 2-Chloro-1-(substituted-phenyl)ethylphosphonic Acids". Bioorganic & Medicinal Chemistry 10 (5): 1281–1290. doi:10.1016/s0968-0896(01)00391-1. PMID 11886791.
- ↑ Alia-Tejacal, I.; Villanueva-Arce, R.; Pelayo-Zaldívar, C.; Colinas-León, M.T.; López-Martínez, V.; Bautista-Baños, S. (2007). "Postharvest physiology and technology of sapote mamey fruit (Pouteria sapota (Jacq.) H.E. Moore & Stearn)". Postharvest Biology and Technology 45 (3): 285–297. doi:10.1016/j.postharvbio.2006.12.024.
- ↑ Li, Yangrui; Solomon, S. (2003). "Ethephon : A versatile growth regulator for sugar cane industry". Sugar Tech 5 (4): 213–223. doi:10.1007/bf02942476.
- ↑ Christopher L. Main and Robert M. Hayes. "Cotton Harvest Aids". University of Tennessee Institute of Agriculture. http://www.utcrops.com/cotton/PDF%20files/W225.pdf.
- ↑ Bill L. Weir and J. M. Gaggero (1982). "Ethephon may hasten cotton boll opening, increase yield". California Agriculture. http://calag.ucanr.edu/archive/?type=pdf&article=ca.v036n09p28.
- ↑ Mansouri, Hakimeh; Salari, Fatemeh; Asrar, Zahra (2013-04-01). "Ethephon application stimulats cannabinoids and plastidic terpenoids production in Cannabis sativa at flowering stage". Industrial Crops and Products 46: 269–273. doi:10.1016/j.indcrop.2013.01.025. ISSN 0926-6690. https://www.sciencedirect.com/science/article/abs/pii/S0926669013000526.
- ↑ Pesticide Information Profiles: Ethephon, Extension Toxicology Net. Sept 1995.
- ↑ "1994 Joint meeting of the FAO panel of experts on pesticide residues in food and the environment." UN Food and Agriculture Organization. 1994.
 |
