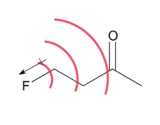
Chemistry:Field effect
A field effect is the polarization of a molecule through space. The effect is a result of an electric field produced by charge localization in a molecule.[1] This field, which is substituent and conformation dependent, can influence structure and reactivity by manipulating the location of electron density in bonds and/or the overall molecule.[2] The polarization of a molecule through its bonds is a separate phenomenon known as induction.[3] Field effects are relatively weak, and diminish rapidly with distance, but have still been found to alter molecular properties such as acidity.[1]
Field sources

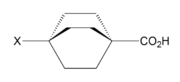
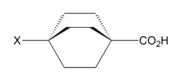
Field effects can arise from the electric dipole field of a bond containing an electronegative atom or electron-withdrawing substituent, as well as from an atom or substituent bearing a formal charge.[1] The directionality of a dipole, and concentration of charge, can both define the shape of a molecule's electric field which will manipulate the localization of electron density toward or away from sites of interest, such as an acidic hydrogen. Field effects are typically associated with the alignment of a dipole field with respect to a reaction center.[5] Since these are through space effects, the 3D structure of a molecule is an important consideration. A field may be interrupted by other bonds or atoms before propagating to a reactive site of interest.[6] Atoms of differing electronegativities can move closer together resulting in bond polarization through space that mimics the inductive effect through bonds.[6] Bicycloheptane and bicyclooctane (seen left) are two compounds in which the change in acidity with substitution was attributed to the field effect.[4] The C-X dipole is oriented away from the carboxylic acid group, and can draw electron density away because the molecule center is empty, with a low dielectric constant, so the electric field is able to propagate with minimal resistance.[5]
Utility of effect
A dipole can align to stabilize or destabilize the formation or loss of a charge, thereby decreasing (if stabilized) or increasing (if destabilized) the activation barrier to a chemical event.[1] Field effects can therefore tune the acidity or basicity of bonds within their fields by donating or withdrawing charge density.[5] With respect to acidity, a common trend to note is that, inductively, an electron-withdrawing substituent in the vicinity of an acidic proton will lower the pKa (i.e. increase the acidity) and, correspondingly, an electron-donating substituent will raise the pKa.[7] The reorganization of charge due to field effects will have the same result. An electric dipole field propagated through the space around, or in the middle of, a molecule in the direction of an acidic proton will decrease the acidity, while a dipole pointed away will increase the acidity and concomitantly elongate the X-H bond.[8] These effects can therefore help to tune the acidity/basicity of a molecule to protonate/deprotonate a specific compound, or enhance hydrogen bond-donor ability for molecular recognition or anion sensing applications.[9][10] Field effects have also been shown in substituted arenes to dominate the electrostatic potential maps, which are maps of electron density used to explain intermolecular interactions.[2]
Evidence for field effects

Localized electronic effects are a combination of inductive and field effects. Due to the similarity in these effects, it is difficult to separate their contributions to the electronic structure of a molecule. There is, however, a large body of literature devoted to developing an understanding of the relative significance of induction and field effects by analyzing related compounds in an attempt to quantify each effect based on the present substituents and molecular geometry.[4][12][13][14][15] For example, the three compounds to the right, all octanes, differ only in the number of linkers between the electron withdrawing group X and an acidic functional group, which are approximately the same spatial distance apart in each compound.[11] It is known that an electron-withdrawing substituent will decrease the pKa of a given proton (i.e. increase the acidity) inductively.[7] If induction was the dominant effect in these compounds, acidity should increase linearly with the number of available inductive pathways (linkers). However, the experimental data shows that effect on acidity in related octanes and cubanes is very similar, and therefore the dominant effect must be through space.[11]

In the cis-11,12-dichloro-9,10-dihydro-9,10-ethano-2-anthroic acid syn and anti isomers seen below and to the left, the chlorines provide a field effect. The concentration of negative charge on each chlorine has a through space effect which can be seen in the relative pKa values.[16] When the chlorines are pointed over the carboxylic acid group, the pKa is higher because loss of a proton is less favorable due to the increase in negative charge in the area. Loss of a proton results in a negative charge which is less stable if there is already an inherent concentration of electrons.[17] This can be attributed to a field effect because in the same compound with the chlorines pointed away from the acidic group the pKa is lower, and if the effect were inductive the conformational position would not matter.[16]


References
- ↑ 1.0 1.1 1.2 1.3 Anslyn, Eric V., Dougherty, Dennis A. (2006). Modern physical organic chemistry. Sausalito, CA: University Science. ISBN 9781891389313. OCLC 55600610.
- ↑ 2.0 2.1 Wheeler, Steven E.; Houk, K. N. (2009). "Through-Space Effects of Substituents Dominate Molecular Electrostatic Potentials of Substituted Arenes". Journal of Chemical Theory and Computation 5 (9): 2301–2312. doi:10.1021/ct900344g. ISSN 1549-9618. PMID 20161573.
- ↑ L., Patrick, Graham (1997). Beginning organic chemistry. Oxford: Oxford University Press. ISBN 978-0198559368. OCLC 37293506.
- ↑ 4.0 4.1 4.2 4.3 Wilcox, Charles F.; Leung, Constance (1968). "Transmission of substituent effects. Dominance of field effects". Journal of the American Chemical Society 90 (2): 336–341. doi:10.1021/ja01004a023. ISSN 0002-7863.
- ↑ 5.0 5.1 5.2 Dewar, Michael J. S.; Grisdale, Patrick J. (1962). "Substituent Effects. I. Introduction". Journal of the American Chemical Society 84 (18): 3539–3541. doi:10.1021/ja00877a023. ISSN 0002-7863.
- ↑ 6.0 6.1 Daley, Richard F., Daley, Sally J. (2005). Organic Chemistry (1.3 ed.). Daley Press.
- ↑ 7.0 7.1 Siggel, Michele R. F.; Streitwieser, Andrew.; Thomas, T. Darrah. (1988). "The role of resonance and inductive effects in the acidity of carboxylic acids". Journal of the American Chemical Society 110 (24): 8022–8028. doi:10.1021/ja00232a011. ISSN 0002-7863.
- ↑ Parveen, Salma; Chandra, Asit K.; Zeegers-Huyskens, Thérèse (2009). "Theoretical Investigation of the Interaction between Fluorinated Dimethyl Ethers (nF = 1−5) and Water: Role of the Acidity and Basicity on the Competition between OH···O and CH···O Hydrogen Bonds". The Journal of Physical Chemistry A 113 (21): 6182–6191. doi:10.1021/jp902244j. ISSN 1089-5639. PMID 19422184. Bibcode: 2009JPCA..113.6182P.
- ↑ Molina, Pedro; Zapata, Fabiola; Caballero, Antonio (2017). "Anion Recognition Strategies Based on Combined Noncovalent Interactions". Chemical Reviews 117 (15): 9907–9972. doi:10.1021/acs.chemrev.6b00814. ISSN 0009-2665. PMID 28665114.
- ↑ Tresca, Blakely W.; Hansen, Ryan J.; Chau, Calvin V.; Hay, Benjamin P.; Zakharov, Lev N.; Haley, Michael M.; Johnson, Darren W. (2015). "Substituent Effects in CH Hydrogen Bond Interactions: Linear Free Energy Relationships and Influence of Anions". Journal of the American Chemical Society 137 (47): 14959–14967. doi:10.1021/jacs.5b08767. ISSN 0002-7863. PMID 26539974.
- ↑ 11.0 11.1 11.2 11.3 11.4 Cole, Thomas W.; Mayers, Carolyn J.; Stock, Leon M. (1974). "Chemistry of the bicyclo[2.2.2]octanes. XV. Dissociation constants of 4-substituted cubane-1-carboxylic acids. Evidence for the field model for the polar effect". Journal of the American Chemical Society 96 (14): 4555–4557. doi:10.1021/ja00821a032. ISSN 0002-7863.
- ↑ (in en) Progress in Physical Organic Chemistry, Volume 13 - Wiley Online Library. Progress in Physical Organic Chemistry. 1981. doi:10.1002/9780470171929. ISBN 9780470171929.
- ↑ Stock, Leon M. (1972). "The origin of the inductive effect". Journal of Chemical Education 49 (6): 400. doi:10.1021/ed049p400. ISSN 0021-9584. Bibcode: 1972JChEd..49..400S.
- ↑ Hansch, Corwin.; Leo, A.; Taft, R. W. (1991). "A survey of Hammett substituent constants and resonance and field parameters". Chemical Reviews 91 (2): 165–195. doi:10.1021/cr00002a004. ISSN 0009-2665.
- ↑ Dewar, Michael J. S.; Grisdale, Patrick J. (1962). "Substituent Effects. IV.1 A Quantitative Theory". Journal of the American Chemical Society 84 (18): 3548–3553. doi:10.1021/ja00877a026. ISSN 0002-7863.
- ↑ 16.0 16.1 16.2 16.3 Grubbs, E.J.; Fitzgerald, R.; Phillips, R.E.; Petty, R. (1971). "The transmission of substituent effects in isomeric dichloroethano-bridged anthracene derivatives". Tetrahedron 27 (5): 935–944. doi:10.1016/s0040-4020(01)92492-5.
- ↑ Pearson, Ralph G. (1963). "Hard and Soft Acids and Bases". Journal of the American Chemical Society 85 (22): 3533–3539. doi:10.1021/ja00905a001. ISSN 0002-7863.