Chemistry:Flavensomycin
From HandWiki
| |
| Names | |
|---|---|
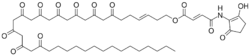
| IUPAC name
[(E)-7,9,11,13,15,17,19,21,23-nonaoxooctatriacont-3-enyl] (E)-4-[(2-hydroxy-5-oxocyclopenten-1-yl)amino]-4-oxobut-2-enoate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C47H65NO14 | |
| Molar mass | 868.030 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Flavensomycin is an antibiotic and fungicide with the molecular formula C47H64NO14.[1][2] Flavensomycin has been first isolated in 1957 from a culture of Streptomyces tanashiensis bacteria.[3]
References
- ↑ 1.0 1.1 "Flavensomycin" (in en). Pubchem.ncbi.NLM.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Flavensomycin#section=3D-Status.
- ↑ Gottlieb, David; Inoue, Yukio (October 1967). "Flavensomycin, an Inhibitor of Enzyme Reactions Involving Hydrogen Transfer". Journal of Bacteriology 94 (4): 844–849. doi:10.1128/jb.94.4.844-849.1967. PMID 4383133.
- ↑ Gottlieb, David (1967). "Flavensomycin". Mechanism of Action. pp. 617–620. doi:10.1007/978-3-642-46051-7_46. ISBN 978-3-642-46053-1.
Further reading
- Gottlieb, David; Shaw, Paul D. (6 December 2012) (in en). Mechanism of Action. Springer Science & Business Media. p. 619. ISBN 978-3-642-46051-7.
 |

