Chemistry:Fosnetupitant
From HandWiki
Short description: Chemical compound
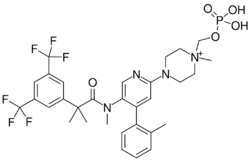 | |
| Clinical data | |
|---|---|
| Other names | 07-PNET, fosnetupitant chloride hydrochloride |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H35F6N4O5P |
| Molar mass | 688.608 g·mol−1 |
Fosnetupitant is a medication used for the treatment of chemotherapy-induced nausea and vomiting. It is a prodrug of netupitant.[1] It is used in combination with palonosetron hydrochloride and formulated as the salt fosnetupitant chloride hydrochloride for intravenous use.[2]
In 2018, the U.S. Food and Drug Administration approved the intravenous formulation of a fixed dose combination of fosnetupitant and palonosetron.[3][4] The combination is also approved for medical use in the European Union[5] and in Canada.[6]
References
- ↑ "Netupitant-palonosetron (NEPA) for Preventing Chemotherapy-induced Nausea and Vomiting: From Clinical Trials to Daily Practice". Current Cancer Drug Targets 22 (10): 806–824. 2022. doi:10.2174/1568009622666220513094352. PMID 35570542.
- ↑ "Fosnetupitant/Palonosetron (Intravenous Route)". Mayo Clinic. https://www.mayoclinic.org/drugs-supplements/fosnetupitant-palonosetron-intravenous-route/description/drg-20425898.
- ↑ "Fosnetupitant". DrugBank. https://go.drugbank.com/drugs/DB14019.
- ↑ "Akynzeo- netupitant and palonosetron capsule Akynzeo- fosnetupitant and palonosetron injection". 28 May 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8e47618e-af46-4d82-94e8-1507c042252d.
- ↑ "Akynzeo EPAR". https://www.ema.europa.eu/en/medicines/human/EPAR/akynzeo.
- ↑ "Akynzeo (netupitant/palonosetron) capsules". Product Insert. Elvium Life Sciences. 31 August 2020. https://pdf.hres.ca/dpd_pm/00057864.PDF.
External links
- "Fosnetupitant". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/fosnetupitant.
 |
