Chemistry:Gedunin
From HandWiki
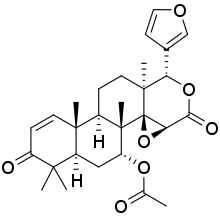
| |
| Names | |
|---|---|
| IUPAC name
Gedunin
| |
| Systematic IUPAC name
[(1S,2R,4S,7S,8S,11R,12R,17R,19R)-7-(furan-3-yl)-1,8,12,16,16-pentamethyl-5,15-dioxo-3,6-dioxapentacyclo[9.8.0.02,4.02,8.012,17]nonadec-13-en-19-yl] acetate | |
Other names
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gedunin is a pentacyclic triterpenoid with the molecular formula C28H34 O7. It is most notably found in Azadirachta indica,[1] but is a constituent of several other plants. Gedunin shows therapeutic potential in the treatment of leukemia,[2] and Parkinson's disease.[3][4]
Natural occurrence
Azadirachta indica[5] is the most notable source of gedunin, but it has also been found in the following plants:
- Cedrela fissilis[6]
- Cedrela odorata[7][8]
- Cedrela salvadorensis[9]
- Entandrophragma angolense[10]
- Khaya grandifoliola[11]
- Melia azedarach[12]
- Toona sinensis[13][14]
- Xylocarpus granatum[15][16]
References
- ↑ Hallur, Gurulingappa; Sivramakrishnan, Apoorba; Bhat, Sujata V. (2002-08-01). "Three New Tetranortriterpenoids from Neem Seed Oil" (in en). Journal of Natural Products 65 (8): 1177–1179. doi:10.1021/np0105174. ISSN 0163-3864. PMID 12193026. https://pubs.acs.org/doi/10.1021/np0105174.
- ↑ Kikuchi, Takashi; Ishii, Koichi; Noto, Taisuke; Takahashi, Akitomo; Tabata, Keiichi; Suzuki, Takashi; Akihisa, Toshihiro (2011-04-25). "Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (neem)". Journal of Natural Products 74 (4): 866–870. doi:10.1021/np100783k. ISSN 1520-6025. PMID 21381696. https://pubmed.ncbi.nlm.nih.gov/21381696.
- ↑ Rane, Anand; Rajagopalan, Subramanian; Ahuja, Manuj; Thomas, Bobby; Chinta, Shankar J.; Andersen, Julie K. (March 2018). "Hsp90 Co-chaperone p23 contributes to dopaminergic mitochondrial stress via stabilization of PHD2: Implications for Parkinson's disease". Neurotoxicology 65: 166–173. doi:10.1016/j.neuro.2018.02.012. ISSN 1872-9711. PMID 29471019.
- ↑ Zhou, Heying; Li, Fengxia; Li, Yanli (November 2022). "Anti-Cancer Activity of Gedunin by Induction of Apoptosis in Human Gastric Cancer AGS Cells". Applied Biochemistry and Biotechnology 194 (11): 5322–5332. doi:10.1007/s12010-022-04001-8. ISSN 1559-0291. PMID 35759172. https://pubmed.ncbi.nlm.nih.gov/35759172/.
- ↑ Chianese, Giuseppina; Yerbanga, Serge R.; Lucantoni, Leonardo; Habluetzel, Annette; Basilico, Nicoletta; Taramelli, Donatella; Fattorusso, Ernesto; Taglialatela-Scafati, Orazio (2010-08-27). "Antiplasmodial Triterpenoids from the Fruits of Neem, Azadirachta indica" (in en). Journal of Natural Products 73 (8): 1448–1452. doi:10.1021/np100325q. ISSN 0163-3864. PMID 20669933. https://pubs.acs.org/doi/10.1021/np100325q.
- ↑ Leite, Ana; Ambrozin, Alessandra; Fernandes, João; Vieira, Paulo; da Silva, Maria; de Albuquerque, Sérgio (December 2008). "Trypanocidal Activity of Limonoids and Triterpenes from Cedrela fissilis" (in en). Planta Medica 74 (15): 1795–1799. doi:10.1055/s-0028-1088323. ISSN 0032-0943. PMID 18991203. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0028-1088323.
- ↑ Campos, Angela M.; Oliveira, Francisco S.; Machado, Maria Iracema L.; Matos, Francisco J.A.; Braz-Filho, Raimundo (January 1991). "Triterpenes from Cedrela odorata" (in en). Phytochemistry 30 (4): 1225–1229. doi:10.1016/S0031-9422(00)95206-3. https://linkinghub.elsevier.com/retrieve/pii/S0031942200952063.
- ↑ Carvalho, Paulo S.; Napolitano, Hamilton B.; Camargo, Ademir J.; Silva, Valter H.C.; Ellena, Javier A.; Rocha, Waldireny C.; Vieira, Paulo C. (January 2012). "X-ray diffraction and theoretical investigation of the Gedunin crystal structure" (in en). Journal of Molecular Structure 1008: 83–87. doi:10.1016/j.molstruc.2011.11.028. https://linkinghub.elsevier.com/retrieve/pii/S0022286011009057.
- ↑ Céspedes, Carlos L.; Calderón, José S.; Lina, Laura; Aranda, Eduardo (2000-05-01). "Growth Inhibitory Effects on Fall Armyworm Spodoptera frugiperda of Some Limonoids Isolated from Cedrela spp. (Meliaceae)" (in en). Journal of Agricultural and Food Chemistry 48 (5): 1903–1908. doi:10.1021/jf990443q. ISSN 0021-8561. PMID 10820113. https://pubs.acs.org/doi/10.1021/jf990443q.
- ↑ Okorie, Domingo A.; Taylor, David A.H. (January 1977). "Triterpenes from the seed of Entandrophragma species" (in en). Phytochemistry 16 (12): 2029–2030. doi:10.1016/0031-9422(77)80123-4. https://linkinghub.elsevier.com/retrieve/pii/0031942277801234.
- ↑ Bickii, Jean; Njifutie, Njikam; Ayafor Foyere, Johnson; Basco, Leonardo K; Ringwald, Pascal (January 2000). "In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae)" (in en). Journal of Ethnopharmacology 69 (1): 27–33. doi:10.1016/S0378-8741(99)00117-8. PMID 10661881. https://linkinghub.elsevier.com/retrieve/pii/S0378874199001178.
- ↑ Khalid, Sami A.; Farouk, Asim; Geary, Timothy G.; Jensen, James B. (February 1986). "Potential antimalarial candidates from African plants: An in vitro approach using Plasmodium falciparum" (in en). Journal of Ethnopharmacology 15 (2): 201–209. doi:10.1016/0378-8741(86)90156-X. PMID 3520157. https://linkinghub.elsevier.com/retrieve/pii/037887418690156X.
- ↑ Mitsui, Kumiko; Saito, Hiroaki; Yamamura, Ryota; Fukaya, Haruhiko; Hitotsuyanagi, Yukio; Takeya, Koichi (2007). "Apotirucallane and Tirucallane Triterpenoids from Cedrela sinensis" (in en). Chemical and Pharmaceutical Bulletin 55 (10): 1442–1447. doi:10.1248/cpb.55.1442. ISSN 0009-2363. PMID 17917286. http://www.jstage.jst.go.jp/article/cpb/55/10/55_10_1442/_article.
- ↑ Mitsui, Kumiko; Saito, Hiroaki; Yamamura, Ryota; Fukaya, Haruhiko; Hitotsuyanagi, Yukio; Takeya, Koichi (2006-09-01). "Hydroxylated Gedunin Derivatives from Cedrela sinensis" (in en). Journal of Natural Products 69 (9): 1310–1314. doi:10.1021/np068021f. ISSN 0163-3864. PMID 16989525. https://pubs.acs.org/doi/10.1021/np068021f.
- ↑ Uddin, Shaikh J.; Nahar, Lutfun; Shilpi, Jamil A.; Shoeb, Mohammad; Borkowski, Tomasz; Gibbons, Simon; Middleton, Moira; Byres, Maureen et al. (August 2007). "Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line In Vitro" (in en). Phytotherapy Research 21 (8): 757–761. doi:10.1002/ptr.2159. PMID 17450509. https://onlinelibrary.wiley.com/doi/10.1002/ptr.2159.
- ↑ Li, Min-Yi; Yang, Xiao-Bo; Pan, Jian-Yu; Feng, Gang; Xiao, Qiang; Sinkkonen, Jari; Satyanandamurty, Tirumani; Wu, Jun (2009-12-28). "Granatumins A−G, Limonoids from the Seeds of a Krishna Mangrove, Xylocarpus granatum" (in en). Journal of Natural Products 72 (12): 2110–2114. doi:10.1021/np900625w. ISSN 0163-3864. PMID 19888743. https://pubs.acs.org/doi/10.1021/np900625w.
 |
