Chemistry:HKUST-1
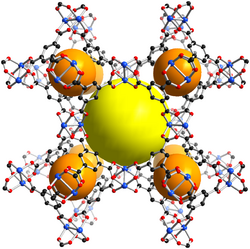
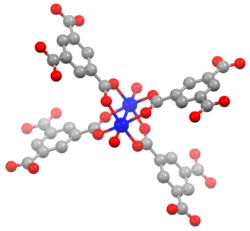
HKUST-1 (HKUST ⇒ Hong Kong University of Science and Technology),[1] which is also called MOF-199,[2] is a material in the class of metal-organic frameworks (MOFs). Metal-organic frameworks are crystalline materials, in which metals are linked by ligands (so-called linker molecules) to form repeating coordination motives extending in three dimensions. The HKUST-1 framework is built up of dimeric metal units, which are connected by benzene-1,3,5-tricarboxylate linker molecules. The paddlewheel unit is the commonly used structural motif to describe the coordination environment of the metal centers and also called secondary building unit (SBU) of the HKUST-1 structure. The paddlewheel is built up of four benzene-1,3,5-tricarboxylate linkers molecules, which bridge two metal centers. One water molecules is coordinated to each of the two metal centers at the axial position of the paddlewheel unit in the hydrated state, which is usually found if the material is handled in air. After an activation process (heating, vacuum), these water molecules can be removed (dehydrated state) and the coordination site at the metal atoms is left unoccupied. This unoccupied coordination site is called coordinatively unsaturated site (CUS) and can be accessed by other molecules.
Structural analogs
Monometallic HKUST-1 analogs
Cu2+ was used as metal center in the first synthesized HKUST-1 material,[1] but the HKUST-1 structure was also obtained with other metals. The oxidation state of most used metals is +II, which results in a neutral overall framework. In the case of trivalent metals (oxidation state +3), the overall framework is positively charged and requires anions to compensate the charge and guarantee charge neutrality.[3][4][5]
| Metal center and
oxidation state |
Year of first
publication |
Alternative
name |
Citation |
|---|---|---|---|
| Cu2+ | 1999 | Cu3BTC2
CuBTC |
[1][6] |
| Mo2+ | 2006 | TUDMOF-1 | [7] |
| Fe2+/3+ | 2007 | [5] | |
| Cr2+ | 2010 | [8] | |
| Ni2+ | 2011 | [9] | |
| Zn2+ | 2011 | [10] | |
| Ru2+/3+ | 2011 | [4] | |
| Mn2+ | 2012 | [11] | |
| Fe2+ | 2012 | [11] | |
| Co2+ | 2012 | [11] | |
| Fe3+ | 2014 | [3] | |
| Ru2+ | 2016 | [12] | |
| Fe2+ | 2019 | [13] |
Mixed-metal HKUST-1 analogs
In addition to monometallic HKUST-1 analogs, several mixed-metal HKUST-1 materials were synthesized, in which two metals are incorporated into the framework structure at crystallographically equivalent positions. The incorporation of two metals can be achieved by using both metals for the synthesis (direct synthesis) or by using post-synthetic metal-exchange. For the post-synthetic metal exchange, a monometallic HKUST-1 material is synthesized in the first step. Subsequently, this monometallic HKUST-1 is suspended in a solution containing the second metal, which results in an exchange of metal centers in the framework leading to a mixed-metal HKUST-1.
| Metal centers and
oxidation states |
Metal ratios
[-] |
Synthesis method | Citation |
|---|---|---|---|
| Cu2+ / Zn2+ | 0.99 : 0.01 | Direct synthesis | [14] |
| 0.99 : 0.01
0.97 : 0.03 0.95 : 0.05 0.90 : 0.10 0.79 : 0.21 |
[15] | ||
| 0.95 : 0.05
0.90 : 0.10 |
Direct synthesis
ball milling (mechanochemical) |
[16] | |
| Cu2+ / Ni2+ | 0.70 : 0.30
0.50 : 0.50 0.20 : 0.80 |
Direct synthesis | [17] |
| Cu2+ / Ru3+ | 0.92 : 0.08 | Direct synthesis | [18] |
| Cu2+ / Ag+ | not reported | Post-synthetic metal-exchange | [19] |
| Cu2+ / Mn2+ | 0.94 : 0.06 | Post-synthetic metal-exchange | [20] |
| Cu2+ / Fe3+ | 0.86 : 0.14 | Post-synthetic metal-exchange | [20] |
| Cu2+ / Co2+ | 0.74 : 0.26 | Post-synthetic metal-exchange | [20] |
| Cu2+ / Pd2+ | 0.91 : 0.09
0.86 : 0.14 0.80 : 0.20 |
Direct synthesis | [21] |
| 0.81 : 0.19
0.59 : 0.41 |
[22] | ||
| Ru2+/3+ / Rh2+ | 0.95 : 0.05
0.89 : 0.11 0.79 : 0.21 0.47 : 0.53 0.24 : 0.76 0.03 : 0.97 |
Direct synthesis | [23] |
| Cu2+ / Fe3+ | 0.69 : 0.31 | Direct synthesis | [24] |
| Cu2+ / Zn2+ / Mo6+ | 0.80 : 0.15 : 0.05
0.70 : 0.15 : 0.15 0.55 : 0.15 : 0.30 |
Direct synthesis
ball milling (mechanochemical) |
[16] |
Theoretically calculated HKUST-1 analogs
Several HKUST-1 analogs have already been synthesized, but several research groups have investigated the properties of the HKUST-1 structure by means of theoretical calculations.[25][26][27][28][29][30] For this purpose, additional metal centers were incorporated into the framework on the theoretical level, which have not been used for the synthesis (e.g. Sc, V, Ti, W, Cd).[27][28] Theoretical study on a mixed-metal HKUST-1 containing Cu in combination with various other metals (e.g. W, Re, Os, Ir, Pt, Au) were also reported, of which several metal combinations have not been synthesized.[29][30]
References
- ↑ Jump up to: 1.0 1.1 1.2 Chui, S. S. (1999-02-19). "A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n". Science 283 (5405): 1148–1150. doi:10.1126/science.283.5405.1148. PMID 10024237. Bibcode: 1999Sci...283.1148C.
- ↑ Britt, D.; Tranchemontagne, D.; Yaghi, O. M. (2008-08-19). "Metal-organic frameworks with high capacity and selectivity for harmful gases" (in en). Proceedings of the National Academy of Sciences 105 (33): 11623–11627. doi:10.1073/pnas.0804900105. ISSN 0027-8424. PMID 18711128. Bibcode: 2008PNAS..10511623B.
- ↑ Jump up to: 3.0 3.1 Sotnik, S. A.; Kolotilov, S. V.; Kiskin, M. A.; Dobrokhotova, Zh. V.; Gavrilenko, K. S.; Novotortsev, V. M.; Eremenko, I. L.; Imshennik, V. K. et al. (April 2014). "Synthesis, crystal structure, and physicochemical properties of the new metal-organic framework — the iron(iii) complex with benzene-1,3,5-tricarboxylate" (in en). Russian Chemical Bulletin 63 (4): 862–869. doi:10.1007/s11172-014-0522-x. ISSN 1066-5285.
- ↑ Jump up to: 4.0 4.1 Kozachuk, Olesia; Yusenko, Kirill; Noei, Heshmat; Wang, Yuemin; Walleck, Stephan; Glaser, Thorsten; Fischer, Roland A. (2011). "Solvothermal growth of a ruthenium metal–organic framework featuring HKUST-1 structure type as thin films on oxide surfaces" (in en). Chemical Communications 47 (30): 8509–11. doi:10.1039/c1cc11107h. ISSN 1359-7345. PMID 21716991. http://xlink.rsc.org/?DOI=c1cc11107h.
- ↑ Jump up to: 5.0 5.1 Xie, Linhua; Liu, Shuxia; Gao, Chaoying; Cao, Ruige; Cao, Jianfang; Sun, Chunyan; Su, Zhongmin (2007-08-15). "Mixed-Valence Iron(II, III) Trimesates with Open Frameworks Modulated by Solvents" (in en). Inorganic Chemistry 46 (19): 7782–7788. doi:10.1021/ic062273m. ISSN 0020-1669. PMID 17696421.
- ↑ Min Wang, Qing; Shen, Dongmin; Bülow, Martin; Ling Lau, Miu; Deng, Shuguang; Fitch, Frank R; Lemcoff, Norberto O; Semanscin, Jessica (2002-09-16). "Metallo-organic molecular sieve for gas separation and purification" (in en). Microporous and Mesoporous Materials 55 (2): 217–230. doi:10.1016/S1387-1811(02)00405-5.
- ↑ Kramer, Markus; Schwarz, Ulrich; Kaskel, Stefan (2006). "Synthesis and properties of the metal-organic framework Mo3(BTC)2 (TUDMOF-1)" (in en). Journal of Materials Chemistry 16 (23): 2245. doi:10.1039/b601811d. ISSN 0959-9428. http://xlink.rsc.org/?DOI=b601811d.
- ↑ Murray, Leslie J.; Dinca, Mircea; Yano, Junko; Chavan, Sachin; Bordiga, Silvia; Brown, Craig M.; Long, Jeffrey R. (2010-06-16). "Highly-Selective and Reversible O 2 Binding in Cr 3 (1,3,5-benzenetricarboxylate) 2" (in en). Journal of the American Chemical Society 132 (23): 7856–7857. doi:10.1021/ja1027925. ISSN 0002-7863. PMID 20481535.
- ↑ Maniam, Palanikumar; Stock, Norbert (2011-06-06). "Investigation of Porous Ni-Based Metal–Organic Frameworks Containing Paddle-Wheel Type Inorganic Building Units via High-Throughput Methods" (in en). Inorganic Chemistry 50 (11): 5085–5097. doi:10.1021/ic200381f. ISSN 0020-1669. PMID 21539354.
- ↑ Feldblyum, Jeremy I.; Liu, Ming; Gidley, David W.; Matzger, Adam J. (2011-11-16). "Reconciling the Discrepancies between Crystallographic Porosity and Guest Access As Exemplified by Zn-HKUST-1" (in en). Journal of the American Chemical Society 133 (45): 18257–18263. doi:10.1021/ja2055935. ISSN 0002-7863. PMID 22011056.
- ↑ Jump up to: 11.0 11.1 11.2 Zhang, Zhenjie; Zhang, Linping; Wojtas, Lukasz; Eddaoudi, Mohamed; Zaworotko, Michael J. (2012-01-18). "Template-Directed Synthesis of Nets Based upon Octahemioctahedral Cages That Encapsulate Catalytically Active Metalloporphyrins" (in en). Journal of the American Chemical Society 134 (2): 928–933. doi:10.1021/ja208256u. ISSN 0002-7863. PMID 22208770.
- ↑ Zhang, Wenhua; Freitag, Kerstin; Wannapaiboon, Suttipong; Schneider, Christian; Epp, Konstantin; Kieslich, Gregor; Fischer, Roland A. (2016-12-19). "Elaboration of a Highly Porous Ru II,II Analogue of HKUST-1" (in en). Inorganic Chemistry 55 (24): 12492–12495. doi:10.1021/acs.inorgchem.6b02038. ISSN 0020-1669. PMID 27989180.
- ↑ Yue, Yanfeng; Arman, Hadi; Chen, Banglin (2019-05-17). "Air-Free Synthesis of a Ferrous Metal-Organic Framework Featuring HKUST-1 Structure and its Mössbauer Spectrum" (in en). Zeitschrift für anorganische und allgemeine Chemie 645 (11): 797–800. doi:10.1002/zaac.201900066. ISSN 1521-3749.
- ↑ Jee, Bettina; Eisinger, Konrad; Gul-E-Noor, Farhana; Bertmer, Marko; Hartmann, Martin; Himsl, Dieter; Pöppl, Andreas (2010-10-07). "Continuous Wave and Pulsed Electron Spin Resonance Spectroscopy of Paramagnetic Framework Cupric Ions in the Zn(II) Doped Porous Coordination Polymer Cu 3− x Zn x (btc) 2" (in en). The Journal of Physical Chemistry C 114 (39): 16630–16639. doi:10.1021/jp105955w. ISSN 1932-7447.
- ↑ Gul-E-Noor, Farhana; Jee, Bettina; Mendt, Matthias; Himsl, Dieter; Pöppl, Andreas; Hartmann, Martin; Haase, Jürgen; Krautscheid, Harald et al. (2012-10-04). "Formation of Mixed Metal Cu 3– x Zn x (btc) 2 Frameworks with Different Zinc Contents: Incorporation of Zn 2+ into the Metal–Organic Framework Structure as Studied by Solid-State NMR" (in en). The Journal of Physical Chemistry C 116 (39): 20866–20873. doi:10.1021/jp3054857. ISSN 1932-7447.
- ↑ Jump up to: 16.0 16.1 Lee, Su-Kyung; Hong, Do-Young; Jeong, Myung-Geun; Yoon, Ji Woong; Bae, Jongyoon; Kim, Young Dok; Chang, Jong-San; Hwang, Young Kyu (2017-11-15). "Trimetallic copper trimesate with isomorphously substituted Mo(VI) and its catalytic properties" (in en). Microporous and Mesoporous Materials 253: 223–232. doi:10.1016/j.micromeso.2017.07.007.
- ↑ Hu, Jue; Yu, Huijing; Dai, Wei; Yan, Xiaoyang; Hu, Xin; Huang, He (2014). "Enhanced adsorptive removal of hazardous anionic dye "congo red" by a Ni/Cu mixed-component metal–organic porous material" (in en). RSC Adv. 4 (66): 35124–35130. doi:10.1039/C4RA05772D. ISSN 2046-2069. http://xlink.rsc.org/?DOI=C4RA05772D.
- ↑ Gotthardt, Meike A.; Schoch, Roland; Wolf, Silke; Bauer, Matthias; Kleist, Wolfgang (2015). "Synthesis and characterization of bimetallic metal–organic framework Cu–Ru-BTC with HKUST-1 structure" (in en). Dalton Transactions 44 (5): 2052–2056. doi:10.1039/C4DT02491E. ISSN 1477-9226. PMID 25518915. http://xlink.rsc.org/?DOI=C4DT02491E.
- ↑ Sun, Zhiguo; Li, Gang; Zhang, Yue; Liu, Hai-ou; Gao, Xionghou (2015-01-10). "Ag–Cu–BTC prepared by postsynthetic exchange as effective catalyst for selective oxidation of toluene to benzaldehyde" (in en). Catalysis Communications 59: 92–96. doi:10.1016/j.catcom.2014.09.047.
- ↑ Jump up to: 20.0 20.1 20.2 Sava Gallis, Dorina F.; Parkes, Marie V.; Greathouse, Jeffery A.; Zhang, Xiaoyi; Nenoff, Tina M. (2015-03-24). "Enhanced O 2 Selectivity versus N 2 by Partial Metal Substitution in Cu-BTC" (in en). Chemistry of Materials 27 (6): 2018–2025. doi:10.1021/cm5042293. ISSN 0897-4756.
- ↑ Zhang, Wenhua; Chen, Zhihao; Al-Naji, Majd; Guo, Penghu; Cwik, Stefan; Halbherr, Olesia; Wang, Yuemin; Muhler, Martin et al. (2016). "Simultaneous introduction of various palladium active sites into MOF via one-pot synthesis: Pd@[Cu 3−x Pd x (BTC) 2 n"] (in en). Dalton Transactions 45 (38): 14883–14887. doi:10.1039/C6DT02893D. ISSN 1477-9226. PMID 27604131. http://xlink.rsc.org/?DOI=C6DT02893D.
- ↑ Guo, Penghu; Froese, Christian; Fu, Qi; Chen, Yen-Ting; Peng, Baoxiang; Kleist, Wolfgang; Fischer, Roland A.; Muhler, Martin et al. (2018-09-20). "CuPd Mixed-Metal HKUST-1 as a Catalyst for Aerobic Alcohol Oxidation" (in en). The Journal of Physical Chemistry C 122 (37): 21433–21440. doi:10.1021/acs.jpcc.8b05882. ISSN 1932-7447. https://publikationen.bibliothek.kit.edu/1000086199/18153018.
- ↑ Heinz, Werner R.; Kratky, Tim; Drees, Markus; Wimmer, Andreas; Tomanec, Ondřej; Günther, Sebastian; Schuster, Michael; Fischer, Roland A. (2019). "Mixed precious-group metal–organic frameworks: a case study of the HKUST-1 analogue [Ru x Rh 3−x (BTC) 2 "] (in en). Dalton Transactions 48 (32): 12031–12039. doi:10.1039/C9DT01198F. ISSN 1477-9226. PMID 31237287. http://xlink.rsc.org/?DOI=C9DT01198F.
- ↑ Bitzer, Johannes; Otterbach, Steffen; Thangavel, Kavipriya; Kultaeva, Anastasia; Schmid, Rochus; Pöppl, Andreas; Kleist, Wolfgang (2020-03-11). "Experimental Evidence for the Incorporation of Two Metals at Equivalent Lattice Positions in Mixed-Metal Metal–Organic Frameworks" (in en). Chemistry – A European Journal 26 (25): 5667–5675. doi:10.1002/chem.201905596. ISSN 0947-6539. PMID 31860147.
- ↑ Ketrat, Sombat; Maihom, Thana; Wannakao, Sippakorn; Probst, Michael; Nokbin, Somkiat; Limtrakul, Jumras (2017-11-20). "Coordinatively Unsaturated Metal–Organic Frameworks M 3 (btc) 2 (M = Cr, Fe, Co, Ni, Cu, and Zn) Catalyzing the Oxidation of CO by N 2 O: Insight from DFT Calculations" (in en). Inorganic Chemistry 56 (22): 14005–14012. doi:10.1021/acs.inorgchem.7b02143. ISSN 0020-1669. PMID 29083883.
- ↑ Maihom, Thana; Probst, Michael; Limtrakul, Jumras (2019). "Computational study of the carbonyl–ene reaction between formaldehyde and propylene encapsulated in coordinatively unsaturated metal–organic frameworks M 3 (btc) 2 (M = Fe, Co, Ni, Cu and Zn)" (in en). Physical Chemistry Chemical Physics 21 (5): 2783–2789. doi:10.1039/C8CP06841K. ISSN 1463-9076. PMID 30667007. Bibcode: 2019PCCP...21.2783M. http://xlink.rsc.org/?DOI=C8CP06841K.
- ↑ Jump up to: 27.0 27.1 Parkes, Marie V.; Sava Gallis, Dorina F.; Greathouse, Jeffery A.; Nenoff, Tina M. (2015-03-26). "Effect of Metal in M 3 (btc) 2 and M 2 (dobdc) MOFs for O 2 /N 2 Separations: A Combined Density Functional Theory and Experimental Study" (in en). The Journal of Physical Chemistry C 119 (12): 6556–6567. doi:10.1021/jp511789g. ISSN 1932-7447.
- ↑ Jump up to: 28.0 28.1 Hu, Tian-ding; Jiang, Yan; Ding, Yi-hong (2019). "Computational screening of metal-substituted HKUST-1 catalysts for chemical fixation of carbon dioxide into epoxides" (in en). Journal of Materials Chemistry A 7 (24): 14825–14834. doi:10.1039/C9TA02455G. ISSN 2050-7488. http://xlink.rsc.org/?DOI=C9TA02455G.
- ↑ Jump up to: 29.0 29.1 Zhang, Qiuju; Cao, Lujie; Li, Baihai; Chen, Liang (2012). "Catalyzed activation of CO2 by a Lewis-base site in W–Cu–BTC hybrid metal organic frameworks" (in en). Chemical Science 3 (9): 2708. doi:10.1039/c2sc20521a. ISSN 2041-6520. http://xlink.rsc.org/?DOI=c2sc20521a.
- ↑ Jump up to: 30.0 30.1 Dong, Xiuqin; Liu, Xiuyu; Chen, Yifei; Zhang, Minhua (March 2018). "Screening of bimetallic M-Cu-BTC MOFs for CO2 activation and mechanistic study of CO2 hydrogenation to formic acid: A DFT study" (in en). Journal of CO2 Utilization 24: 64–72. doi:10.1016/j.jcou.2017.11.014.
 |