Chemistry:Hadrucalcin
Hadrucalcin is a peptide toxin from the venom of the scorpion Hadrurus gertschi.[1] Hadrucalcin modifies the Ryanodine receptor channels RyR1 and RyR2, found in the sarcoplasmic reticulum, to a long-lasting subconductance state, thus inducing the release of calcium from the sarcoplasmic reticulum.[2]
Source and etymology
Hadrucalcin (HdCa, alternative spelling: Hadrucalcine) is obtained from the venom gland of the scorpion Hadrurus gertschi,[2] endemic to Guerrero, Mexico.[3] Hadrucalcin is named after the scorpion that produces the peptide and its similarity to other toxins of the scorpion calcin family.[2]
Chemistry
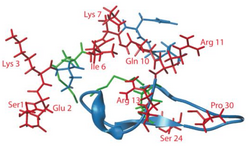
Hadrucalcin is a peptide toxin from the scorpion calcin family.[1] The mature hadrucalcin peptide is composed of 35 amino acids, whereas other members of the scorpion calcin family are composed of 33 amino acids.[2] Based on the high degree of sequence similarity with maurocalcin, it is predicted that hadrucalcin folds along an inhibitor cystine knot motif,[2] with disulfide bonds between positions 5 and 19, 12 and 23 and 18 and 34 of the mature peptide.[1] Hadrucalcin has a molecular weight of 4190.5 Da.[2] The most notable difference between hadrucalcin and other members of the scorpion calcin family is that hadrucalcin lacks two positive charged lysine residues (at positions 8 and 22 in other scorpion calcine toxins),[2] that were previously thought to be important for affinity with RyR1.[4] Instead, Hadrucalcin has two Lysine residues at positions 3 and 7 of the mature peptide, near the otherwise neutral N-terminal, resulting in a relatively low degree of amphiphilicity.[2]
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 21 | 32 | 33 | 34 | 35 | |
| HdCa[1] | S | E | K | D | C | I | K | H | L | Q | R | C | R | E | N | K | D | C | C | S | K | K | C | S | R | R | G | T | N | P | E | K | R | C | R |
| MCa[5] | G | D | C | L | P | H | L | K | L | C | K | E | N | K | D | C | C | S | K | K | C | K | R | R | G | T | N | I | E | K | R | C | R | ||
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 21 | 32 | 33 |
Target and Mode of Action
Hadrucalcin targets ryanodine receptor channels RyR1 and RyR2, found in the sarcoplasmic reticulum of skeletal muscle cells and cardiac muscle cells respectively.[1] By inducing a long-lasting subconductance state in RyR1 (35% of full conductance) and RyR2 (50% of full conductance), hadrucalcin increases the total ion flow over these channels.[2] Within a few seconds, hadrucalcin is able to permeate the cell membrane of ventricular myocytes and induce the release of calcium from the sarcoplasmic reticulum.[2] Despite functional effects on both channels, hadrucalcin only enhances the binding of ryanodine to RyR1 (EC50 = 37.8nM Hadrucalcin), but not to RyR2, suggesting the binding sites for hadrucalcin are structurally different in RyR1 and RyR2.[2] The exact interacting sites of hadrucalcin on the ryanodine receptors are unknown.
Therapeutic Use
Maurocalcin is the first proven example of a scorpion toxin that can act as a vector for large compounds.[6] Hadrucalcin appears to be an even more potent cell penetrating peptide.[7] A Hadrucalcin derivative has been used as a carrier for nanobiosensors.[8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Hadrucalcin precursor - Hoffmannihadrurus gertschi (Scorpion)". https://www.uniprot.org/uniprot/B8QG00.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Schwartz EF, Capes EM, Diego-García E, et al. Characterization of hadrucalcin, a peptide from Hadrurus gertschi scorpion venom with pharmacological activity on ryanodine receptors. Br J Pharmacol. 2009;157(3):392-403. doi:10.1111/j.1476-5381.2009.00147.x.
- ↑ Soleglad ME. The Taxonomy of the Genus Hadrurus Based on Chela Trichobothria (Scorpionida: Vejovidae). J Arachnol. 1976;3:113-134. doi:10.2307/3705291.
- ↑ Lee CW, Lee EH, Takeuchi K, et al. Molecular basis of the high-affinity activation of type 1 ryanodine receptors by imperatoxin A. Biochem J. 2004;377(Pt 2):385-94. doi:10.1042/BJ20031192.
- ↑ "UniProt". https://www.uniprot.org/uniprot/P60254..
- ↑ Estève E, Mabrouk K, Dupuis A, et al. Transduction of the scorpion toxin maurocalcine into cells. Evidence that the toxin crosses the plasma membrane. J Biol Chem. 2005;280(13):12833-9. doi:10.1074/jbc.M412521200.
- ↑ Tisseyre C, Bahembera E, Dardevet L, Sabatier J-M, Ronjat M, De Waard M. Cell penetration properties of a highly efficient mini maurocalcine Peptide. Pharmaceuticals (Basel). 2013;6(3):320-39. doi:10.3390/ph6030320.
- ↑ Zamaleeva AI, Collot M, Bahembera E, et al. Cell-Penetrating Nanobiosensors for Pointillistic Intracellular Ca 2+ - Transient Detection. 2014.
 |