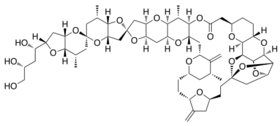
Chemistry:Halichondrin B
| |
| |
| Names | |
|---|---|
| IUPAC name
(1S,2S,2′S,3S,3aS,3a′S,5R,6S,7S,7′S,7aS,7a′S,9S,12S,14R,16R,18S,20S,22R,26R,28S,29S,30R,34R,37S,39R,40S,41R,43R,44S)-7,7′,14′′,29′′-tetramethyl-8′′,15′′-dimethylidene-2-(1,3,4-trihydroxybutyl)decahydro-3′H,32′′H-dispiro[furo[3,2-b]pyran-5,5′-furo[3,2-b]pyran-2′,24′′-[2,19,23,27,31,38,42,45,47,48,49]undecaoxaundecacyclo[32.9.2.1~3,40~.1~3,41~.1~6,9~.1~12,16
~.0~18,30~.0~20,28~.0~22,26~.0~37,44~.0~39,43~]nonatetracontan]-32′′-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C60H86O19 | |
| Molar mass | 1111.329 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Halichondrin B is a polyether macrolide originally isolated from the marine sponge Halichondria okadai by Hirata and Uemura in 1986.[1] In the same report, these authors also reported the exquisite anticancer activity of halichondrin B against murine cancer cells both in culture and in in vivo studies. Halichondrin B was highly prioritized for development as a novel anticancer therapeutic by the United States National Cancer Institute[2] and, in 1991, was the original test case for identification of mechanism of action (in this case, tubulin-targeted mitotic inhibitor) by NCI's then-brand-new "60-cell line screen".[3][4]
The complete chemical synthesis of halichondrin B was achieved by Yoshito Kishi and colleagues at Harvard University in 1992,[5] an achievement that ultimately enabled the discovery and development of the structurally simplified and pharmaceutically optimized analog eribulin (E7389, ER-086526, NSC-707389).[6][7] Eribulin was approved by the U.S. Food and Drug Administration on November 15, 2010, to treat patients with metastatic breast cancer who have received at least two prior chemotherapy regimens for late-stage disease, including both anthracycline- and taxane-based chemotherapies.[8] Eribulin is marketed by Eisai Co. under the tradename Halaven.
More recently, another halichondrin B derivative, E7130, was synthesized in collabortaion by Eisai Co. and the Kishi lab, and has entered clinical trials.[9][10][11]
Biosynthesis
While a producer organism for Halichondrin B has never been isolated in pure culture, the structural features of Halichondrin B, such as the 'odd-even' rule of methylation, and the abundance of oxygen heterocycles, suggest it is a product of dinoflagellate polyether metabolism[12] In support of this conjecture, the known dinoflagellate toxin okadaic acid was isolated from the same species of sponge.[13] But, halichondrin B is not found in the geographically and relatively phylogenetically close sponges H. panicea or H. japonica which are found in similar tide pools in Japan as Halichondria okadai.[14] In constrast, halichondrins have been reported from geographically and phylogenetically distant sponges to Halichondria okadai, including Axinella sp.[15] and Phakellia carteri,[16] and Lissodendoryx. Aquaculture of the New Zealand sponge Lissodendoryx n. sp. 1 over at least 7 years, distant from its original range (at ~10 m depth nearby Wellington vs its native range ~90 m deep off the Kaikōura Peninsula), established it could produce halichondrin B at a relatively high yield over a timecourse of years, suggesting that halichondrins were being produced by vertically inherited symbionts, rather than being concentrated from a dietary source present in the environment.[17][18][19] In fact, the bulk of halichondrin B used by the NCI for its theraputic evaluation, was isolated from New Zealand Lissodendoryx rather than from Halichondria okadai.[19]
See also
- Altohyrtin A
References
- ↑ "Halichondrins - antitumor polyether macrolides from a marine sponge". Pure Appl. Chem. 58 (5): 701–710. 1986. doi:10.1351/pac198658050701.
- ↑ "Success Story: Halichondrin B (NSC 609395) E7389 (NSC 707389)". Developmental Therapeutics Program, National Cancer Institute. https://dctd.cancer.gov/programs/dtp.
- ↑ "NCI-60 DTP Human Tumor Cell Line Screen". Developmental Therapeutics Program, National Cancer Institute. http://www.dtp.nci.nih.gov/branches/btb/ivclsp.html.
- ↑ "Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data". J. Biol. Chem. 266 (24): 15882–9. August 1991. doi:10.1016/S0021-9258(18)98491-7. PMID 1874739.
- ↑ "Total synthesis of halichondrin B and norhalichondrin B". J. Am. Chem. Soc. 114 (8): 3162–3164. 1992. doi:10.1021/ja00034a086.
- ↑ "In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B". Cancer Res. 61 (3): 1013–21. February 2001. PMID 11221827.
- ↑ "Discovery of E7389, a fully synthetic macrocyclic ketone analogue of halichondrin B". Anticancer agents from natural products. Washington, DC: Taylor & Francis. 2005. ISBN 978-0-8493-1863-4.
- ↑ "FDA approves new treatment option for late-stage breast cancer" (Press release). USFDA. 2010-11-15. Archived from the original on November 17, 2010. Retrieved November 15, 2010.
- ↑ Kawano, Satoshi; Ito, Ken; Yahata, Kenzo; Kira, Kazunobu; Abe, Takanori; Akagi, Tsuyoshi; Asano, Makoto; Iso, Kentaro et al. (2019-06-17). "A landmark in drug discovery based on complex natural product synthesis". Scientific Reports 9 (1). doi:10.1038/s41598-019-45001-9. ISSN 2045-2322. PMID 31209263. PMC 6572832. https://www.nature.com/articles/s41598-019-45001-9.pdf. Retrieved 2025-05-28.
- ↑ Kaburagi, Yosuke; Kira, Kazunobu; Yahata, Kenzo; Iso, Kentaro; Sato, Yuki; Matsuura, Fumiyoshi; Ohashi, Isao; Matsumoto, Yasunobu et al. (2024-04-12). "Ten-Gram-Scale Total Synthesis of the Anticancer Drug Candidate E7130 to Supply Clinical Trials". Organic Letters 26 (14): 2837–2842. doi:10.1021/acs.orglett.3c03663. ISSN 1523-7060. https://pubs.acs.org/doi/10.1021/acs.orglett.3c03663. Retrieved 2025-05-28.
- ↑ Sasaki, Takeo; Yahata, Kenzo; Isomura, Minetaka; Ohashi, Isao; Fukuyama, Takashi; Miyashita, Yusuke; Watanabe, Yuzo; Murai, Norio et al. (2024-06-21). "What Does It Take to Develop Structurally Complex Molecules by Total Synthesis? Rapid Process Development and GMP Manufacturing of E7130 Drug Substance for First-in-Human Clinical Study". Organic Process Research & Development 28 (6): 2077–2089. doi:10.1021/acs.oprd.4c00016. ISSN 1083-6160. https://pubs.acs.org/doi/10.1021/acs.oprd.4c00016. Retrieved 2025-05-28.
- ↑ Van Wagoner, Ryan M.; Satake, Masayuki; Wright, Jeffrey L. C. (2014-06-16). "Polyketide biosynthesis in dinoflagellates: what makes it different?". Natural Product Reports (Royal Society of Chemistry (RSC)) 31 (9): 1101–37. doi:10.1039/c4np00016a. ISSN 0265-0568. PMID 24930430.
- ↑ Tachibana, Kazuo; Scheuer, Paul J.; Tsukitani, Yasumasa; Kikuchi, Hiroyuki; Van Engen, Donna; Clardy, Jon; Gopichand, Yalamanchili; Schmitz, Francis J. (1981). "Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria". Journal of the American Chemical Society (American Chemical Society (ACS)) 103 (9): 2469–2471. doi:10.1021/ja00399a082. ISSN 0002-7863.
- ↑ Abe, Takahiro; Sahin, Fatma Pinar; Akiyama, Kiyotaka; Naito, Takayuki; Kishigami, Mizoe; Miyamoto, Kenji; Sakakibara, Yasufumi; Uemura, Daisuke (2012-04-23). "Construction of a Metagenomic Library for the Marine Sponge Halichondria okadai". Bioscience, Biotechnology, and Biochemistry 76 (4): 633–639. doi:10.1271/bbb.110533. ISSN 0916-8451.
- ↑ Pettit, George R.; Herald, Cherry L.; Boyd, Michael R.; Leet, John E.; Dufresne, Claude; Doubek, Dennis L.; Schmidt, Jean M.; Cerny, Ronald L. et al. (1991). "Antineoplastic agents. 219. Isolation and structure of the cell growth inhibitory constituents from the western Pacific marine sponge Axinella sp". Journal of Medicinal Chemistry 34 (11): 3339–3340. doi:10.1021/jm00115a027. ISSN 0022-2623. https://pubs.acs.org/doi/abs/10.1021/jm00115a027. Retrieved 2025-06-02.
- ↑ Pettit, George R.; Tan, Rui; Gao, Feng; Williams, Michael D.; Doubek, Dennis L.; Boyd, Michael R.; Schmidt, Jean M.; Chapuis, Jean Charles et al. (1993). ": Isolation and structure of halistatin 1 from the eastern Indian Ocean marine sponge Phakellia carteri". The Journal of Organic Chemistry 58 (9): 2538–2543. doi:10.1021/jo00061a030. ISSN 0022-3263. https://pubs.acs.org/doi/abs/10.1021/jo00061a030. Retrieved 2025-06-02.
- ↑ Munro, Murray H.G.; Blunt, John W.; Dumdei, Eric J.; Hickford, Sarah J.H.; Lill, Rachel E.; Li, Shangxiao; Battershill, Christopher N.; Duckworth, Alan R. (1999). "The discovery and development of marine compounds with pharmaceutical potential". Journal of Biotechnology 70 (1-3): 15–25. doi:10.1016/S0168-1656(99)00052-8. https://linkinghub.elsevier.com/retrieve/pii/S0168165699000528. Retrieved 2025-06-02.
- ↑ Hart, Joanne B.; Lill, Rachel E.; Hickford, Sarah J.H.; Blunt, John W.; Munro, Murray H.G. (2000-01-01). "The Halichondrins: Chemistry, Biology, Supply and Delivery". Drugs from the Sea. Basel ; New York: Karger Medical and Scientific Publishers. p. 134-153. ISBN 978-3-8055-7098-5. https://pharmacy.hebmu.edu.cn/trywhx/resources/43/202009/1599723823714021067.pdf#page=140.
- ↑ 19.0 19.1 Newman, David J; Cragg, Gordon M; Battershill, Christopher N (2009). "Therapeutic agents from the sea: biodiversity, chemo-evolutionary insight and advances to the end of Darwin’s 200th year". Diving and Hyperbaric Medicine (South Pacific Underwater Medicine Society (Incorporated in Victoria) AO020660B and the European Underwater and Baromedical Society) 39 (4): 216-225. http://www.dhmjournal.com/images/Journals/39/DHM_Vol39_No4.pdf#page=30.
 |
