Chemistry:Hydantoic acid
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
N-Carbamoylglycine
| |
| Systematic IUPAC name
(Carbamoylamino)acetic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H6N2O3 | |
| Molar mass | 118.092 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
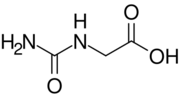
Hydantoic acid is an acid with the chemical formula C3H6N2O3. Its molecule contains a total of 13 bonds including seven non-H bonds, two multiple bonds, two rotatable bonds, two double bonds, one carboxylic acid (aliphatic), one urea derivative, and one hydroxyl group.[1] It can be obtained from uric acid[2] as well as from glycine with urea in the presence of alkali. [3]
References
- ↑ "Chemical Structure of Hydantoic acid (C3H6N2O3)". MolInstincts Chemical Database. https://www.molinstincts.com/chemical-structure/Hydantoic-acid-cstr-CT1000032912.html.
- ↑ Peter Wallwork Latham (1884). On the Formation of Uric Acid in Animals: Its Relation to Gout and Gravel, Together with an Explanation of the Therapeutic Effects of Some of the Remedies Used in the Treatment of Those Disorders. Deighton, Bell. p. 14. https://archive.org/details/onformationuric00lathgoog.
- ↑ V.K. Ahluwalia; R. Aggarwal, V.K. Ahluwalia. Comprehensive Practical Organic Chemistry: Preparations And Quantitative Analysis. Universities Press. p. 227. ISBN 978-81-7371-475-7. https://books.google.com/books?id=mnsKyupepQEC&pg=PA227.
 |
