Chemistry:Hydroxyethylethylenediaminetriacetic acid
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
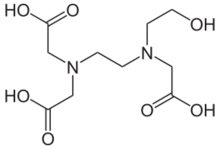
N-(Carboxymethyl)-N-{2-[N-(2-hydroxyethyl)glycino]ethyl}glycine
| |
| Systematic IUPAC name
2,2′-({2-[(2-Hydroxyethyl)(carboxymethyl)amino]ethyl}azanediyl)diacetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H18N2O7 | |
| Molar mass | 278.261 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxyethylethylenediaminetriacetic acid also known as HEDTA is a tricarboxylic acid and amine. It is a hexadentate ligand. It can chelate or form salts with many metals.[1]
References
- ↑ Chaberek, S.; Martell, Arthur E. (March 1955). "Interaction of Divalent Metal Ions with N-Hydroxyethylethylenediaminetriacetic Acid". Journal of the American Chemical Society 77 (6): 1477–1480. doi:10.1021/ja01611a022.
 |

