Chemistry:Hydroxymatairesinol
From HandWiki
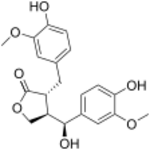
| |
| Names | |
|---|---|
| IUPAC name
(7′S,8β,8′α)-4,4′,7′-Trihydroxy-3,3′-dimethoxylignano-9,9′-lactone
| |
| Systematic IUPAC name
(3R,4R)-4-[(S)-Hydroxy(4-hydroxy-3-methoxyphenyl)methyl]-3-[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H22O7 | |
| Molar mass | 374.389 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Hydroxymatairesinol (HMR) is a lignan found in Norway spruce (Picea abies).[1] It is an enterolactone precursor with anticancer activities. In rats, HMR decreased the volume of induced tumours and stabilised established tumours, as well as preventing the development of new tumours.[1] It has also shown anti-oxidant properties in vitro.[1]
HMR's chemical structure is similar to matairesinol.[2] At high concentrations, HMR has estrogenic properties, which are considerably weaker than those of estradiol.[2]
References
- ↑ 1.0 1.1 1.2 Saarinen, NM; Warri, A; Makela, SI et al. (2000). "Hydroxymatairesinol, a novel enterolactone precursor with antitumor properties from coniferous tree (Picea abies).". Nutrition and Cancer 36 (2): 207–16. doi:10.1207/S15327914NC3602_10. PMID 10890032.
- ↑ 2.0 2.1 Cosentino, M; Marino, F; Ferrari, M et al. (August 2007). "Estrogenic activity of 7-hydroxymatairesinol potassium acetate (HMR/lignan) from Norway spruce (Picea abies) knots and of its active metabolite enterolactone in MCF-7 cells.". Pharmacological Research 56 (2): 140–7. doi:10.1016/j.phrs.2007.05.001. PMID 17572100.
 |

