Chemistry:Imidazolate
From HandWiki
Short description: Ion
| |
| Names | |
|---|---|
| IUPAC name
Imidazolate
| |
| Other names
Imidazolide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| C3H3N2 | |
| Molar mass | 67.070 |
| Acidity (pKa) | 14.05[1] |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
67.8 kJ·mol−1 (16.2 kcal·mol−1) Gas phase.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
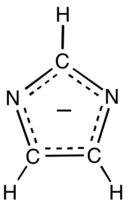
Imidazolate (C3H3N−2) is the conjugate base of imidazole. It is a nucleophile and a strong base. The free anion has C2v symmetry. Imidazole has a pKa of 14.05,[1] so the deprotonation of imidazole (C3H3N2H) requires a strong base.
Occurrence

Imidazolate is a common bridging ligand in coordination chemistry. In the zeolitic imidazolate frameworks, the metals are interconnected via imidazolates.[4][5] In the enzyme superoxide dismutase, imidazolate links copper and zinc centers.
References
- ↑ 1.0 1.1 WALBA, HAROLD; ISENSEE, ROBERT W. (August 1961). "Acidity Constants of Some Arylimidazoles and Their Cations". The Journal of Organic Chemistry 26 (8): 2789–2791. doi:10.1021/jo01066a039.
- ↑ Gutowski, Keith E.; Rogers, Robin D.; Dixon, David A. (May 2007). "Accurate Thermochemical Properties for Energetic Materials Applications. II. Heats of Formation of Imidazolium-, 1,2,4-Triazolium-, and Tetrazolium-Based Energetic Salts from Isodesmic and Lattice Energy Calculations". The Journal of Physical Chemistry B 111 (18): 4788–4800. doi:10.1021/jp066420d. PMID 17388432.
- ↑ PDB: 3CQQ; "Structures of the G85R variant of SOD1 in familial amyotrophic lateral sclerosis". J. Biol. Chem. 283 (23): 16169–77. June 2008. doi:10.1074/jbc.M801522200. PMID 18378676.
- ↑ Phan, A.; Doonan, C. J.; Uribe-Romo, F. J.; Knobler, C. B.; O'Keeffe, M.; Yaghi, O. M. "Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks" Acc. Chem. Res. 2010, 43, 58-67. doi:10.1021/ar900116g
- ↑ Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M., "Metal Azolate Frameworks: From Crystal Engineering to Functional Materials", Chem. Rev. 2012, vol. 112, pp. 1001-1033. doi:10.1021/cr200139g
 |
