Chemistry:Iridodial
From HandWiki
| |

| |
| Names | |
|---|---|
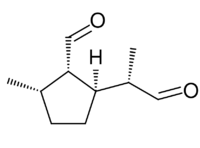
| Preferred IUPAC name
(1S,2S,5R)-2-Methyl-5-[(2S)-1-oxopropan-2-yl]cyclopentane-1-carbaldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H16O2 | |
| Molar mass | 168.236 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Iridodial is an iridoid. It is produced from 8-oxogeranial by the enzyme iridoid synthase (IS).[1] Iridodial is one of the substrates for the enzyme iridoid oxidase (IO) which produces 7-deoxyloganetic acid.
Although it may not be known at this time whether the compound proper iridodial is actually present in natural plant species or not, the use of analogous ion iridodial cation is mentioned in several papers on iridoid biosyntheses in plant species. [2][3]
References
- ↑ Geu-Flores, Fernando; Sherden, Nathaniel H.; Courdavault, Vincent; Burlat, Vincent; Glenn, Weslee S.; Wu, Cen; Nims, Ezekiel; Cui, Yuehua et al. (2012). "An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis". Nature 492 (7427): 138–42. doi:10.1038/nature11692. PMID 23172143. Bibcode: 2012Natur.492..138G.
- ↑ Uesato (1984). "Biosynthetic pathway of iridoid glucosides in Gardenia jasminoides f. grandiflora cell suspension cultures after iridodial cation formation". Tetrahedron Letters 25 (5): 573–576. doi:10.1016/S0040-4039(00)99941-6.
- ↑ Sampaio-Santos (2001). "Biosynthesis significance of iridoids in chemosystematics.". Journal of the Brazilian Chemical Society (12): 144–153.
 |
