Chemistry:Isomalathion
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
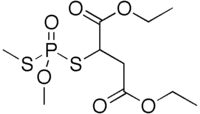
Diethyl {[methoxy(methylsulfanyl)phosphoryl]sulfanyl}butanedioate | |
| Other names
S-Methylmalathion
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H19O6PS2 | |
| Molar mass | 330.36 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Isomalathion is an impurity found in some batches of malathion. Whereas the structure of malation is, generically, RSP(S)(OCH3)2, the connectivity of isomalathion is RSPO(SCH3)(OCH3). It arises by heating malathion. Being significantly more toxic to humans than malathion, it has resulted in human poisonings.[1]
In 1976, numerous malaria workers in Pakistan were poisoned by isomalathion.[2] It is an inhibitor of carboxyesterase.
References
- ↑ Rengasamy, S.; Parmer, Balraj S. (1988). "Investigation of some factors influencing isomalathion formation in malathion products". Journal of Agricultural and Food Chemistry 36 (5): 1025–1030. doi:10.1021/jf00083a030.
- ↑ Baker EL; Warren M; Zack M et al. (1978). "Epidemic malathion poisoning in Pakistan malaria workers". Lancet 1 (8054): 31–4. doi:10.1016/S0140-6736(78)90375-6. PMID 74508.
 |
