Chemistry:Isopropylmalic acid
From HandWiki
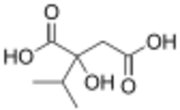 2-Isopropylmalic acid
| |
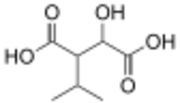 3-Isopropylmalic acid
| |
| Names | |
|---|---|
| IUPAC names
3-Isopropylmalic acid
2-Hydroxy-3-isopropylsuccinic acid | |
| Other names
Isopropylmalate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| DrugBank |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H12O5 | |
| Molar mass | 176.168 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Isopropylmalic acid (isopropylmalate) is an intermediate in the biosynthesis of leucine, synthesized from oxoisovalerate by 2-isopropylmalate synthase and converted into isopropyl-3-oxosuccinate by 3-isopropylmalate dehydrogenase. Two isomers are important, the 2- and 3-isopropyl derivatives, and these are interconverted by isopropylmalate dehydratase.
This article does not cite any external source. HandWiki requires at least one external source. See citing external sources. (2021) (Learn how and when to remove this template message) |
 |

