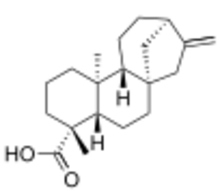
Chemistry:Kaurenoic acid
| |
| Names | |
|---|---|
| IUPAC name
5β,8α,9β,10α,13α-Kaur-16-en-18-oic acid
| |
| Systematic IUPAC name
(4R,4aS,6aS,9R,11aR,11bS)-4,11b-Dimethyl-8-methylidenetetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 10784819 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H30O2 | |
| Molar mass | 302.45 |
| Pharmacology | |
| 1=ATC code }} | M09AX05 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Kaurenoic acid (ent-kaur-16-en-19-oic acid or Kauren-19-oic acid) is a diterpene with antibacterial activity against Gram-positive bacteria. However its low solubility and blood lytic activity on erythrocytes might make it a poor pharmaceutical candidate.[1] Kaurenoic acid also has uterine relaxant activity via calcium blockade and opening ATP-sensitive potassium channels.
Kaurenoic acid is found in several plants such as Copaifera. It is a potential biomarker for the presence of sunflower in foods.[2]
Medical use
Kaurenoic acid has been studied for its medicinal properties and seems to have anti-inflammatory, antiulcerogenic, antitumor, antinociceptive, antimelanoma, antitilipoperoxidation, antioxidant and antimicrobial properties.[3]
Kaurenoic acid decreases leukocyte migration. It seems to inhibit histamine and serotonin pathways, in addition to antiprotozoal activities against Trypanosoma cruzi[4][5] and Leishmania amazonensis.[6]
References
- ↑ Vieira, Henriete S.; Takahashi, Jacqueline A.; Oliveira, Alaíde B. de; Chiari, Egler; Boaventura, Maria Amélia D. (2002). "Novel Derivatives of Kaurenoic Acid". Journal of the Brazilian Chemical Society 13 (2): 151–157. doi:10.1590/S0103-50532002000200004. ISSN 0103-5053.
- ↑ "Showing Compound Kaurenoic acid (FDB021671) - FooDB". https://foodb.ca/compounds/FDB021671.
- ↑ Rocha, Silvia Maria Machado da; Cardoso, Plínio Cerqueira dos Santos; Bahia, Marcelo de Oliveira; Pessoa, Claudia do Ó; Soares, Paulo Cardoso; Rocha, Simone Machado da; Burbano, Rommel Mário Rodríguez; Rocha, Carlos Alberto Machado da (1 June 2019). "Effect of the kaurenoic acid on genotoxicity and cell cycle progression in cervical cancer cells lines". Toxicology in Vitro 57: 126–131. doi:10.1016/j.tiv.2019.02.022. ISSN 0887-2333. PMID 30822460. http://www.sciencedirect.com/science/article/pii/S0887233318308476. Retrieved 24 January 2021.
- ↑ Silva, Matheus L.; Costa‐Silva, Thais A.; Antar, Guilherme M.; Tempone, Andre G.; Lago, João Henrique G. (October 2021). "Front Cover: Chemical Constituents from Aerial Parts of Baccharis sphenophylla and Effects against Intracellular Forms of Trypanosoma cruzi (Chem. Biodiversity 10/2021)" (in en). Chemistry & Biodiversity 18 (10). doi:10.1002/cbdv.202100695. ISSN 1612-1872. https://onlinelibrary.wiley.com/doi/10.1002/cbdv.202100695.
- ↑ da Costa-Silva, Thais A.; Silva, Matheus L.; Antar, Guilherme M.; Tempone, Andre G.; Lago, João Henrique G. (2021-12-01). "Ent-kaurane diterpenes isolated from n-hexane extract of Baccharis sphenophylla by bioactivity-guided fractionation target the acidocalcisomes in Trypanosoma cruzi" (in en). Phytomedicine 93: 153748. doi:10.1016/j.phymed.2021.153748. ISSN 0944-7113. PMID 34628240. https://www.sciencedirect.com/science/article/pii/S0944711321002919.
- ↑ al, D. Kian et (2018). "Trypanocidal activity of copaiba oil and kaurenoic acid does not depend on macrophage killing machinery |". Biomedicine & Pharmacotherapy 103: 1294–1301. doi:10.1016/j.biopha.2018.04.164. PMID 29864911. https://paperpanda.app/viewer?doi=10.1016%2Fj.biopha.2018.04.164&token=WzI2ODY0NjEsIjEwLjEwMTYvai5iaW9waGEuMjAxOC4wNC4xNjQiXQ.aSN7hK_A6gZEM6lXBdtB-Iw5VTk. Retrieved 24 January 2021.
 |

