Chemistry:Kelliphite
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
6,6′-{[1,1′-Biphenyl]-2,2′-diylbis(oxy)}bis(4,8-di-tert-butyl-1,2,10,11-tetramethyl-6H-dibenzo[d,f][1,3,2]dioxaphosphepine) | |
| Other names
Kelliphite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C60H72O6P2 | |
| Molar mass | 951.178 g·mol−1 |
| organic solvents | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
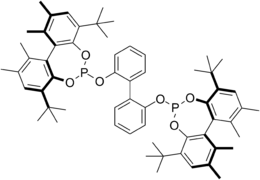
Kelliphite is an acronym for the organophosphorus compound 6,6'-[(1,1'-Biphenyl-2,2'-diyl)bis(oxy)]bis[4,8-di-tbutyl-1,2,10,11-tetramethyl]dibenzo[d,f][1,3,2]dioxaphosphepin. This chiral ligand is widely used in asymmetric synthesis.[1][2] In one example, this ligand is used to form a rhodium complex to catalyze asymmetric hydroformylation of prochiral olefins. It has been shown that high substrate concentrations as well as a wide variety of functional groups are tolerated. [3]
References
- ↑ Clark, Thomas P.; Landis, CR; Freed, SL; Klosin, J; Abboud, KA (2005). "Highly Active, Regioselective, and Enantioselective Hydroformylation with Rh Catalysts Ligated by Bis-3,4-diazaphospholanes". J. Am. Chem. Soc. 127 (14): 5040–2. doi:10.1021/ja050148o. PMID 15810837.
- ↑ Cobley, Christopher J.; Gardner, K; Klosin, J; Praquin, C; Hill, C; Whiteker, GT; Zanotti-Gerosa, A; Petersen, JL et al. (2004). "Synthesis and Application of a New Bisphosphite Ligand Collection for Asymmetric Hydroformylation of Allyl Cyanide". J. Org. Chem. 69 (12): 4031–40. doi:10.1021/jo040128p. PMID 15176828. https://figshare.com/articles/journal_contribution/3335989.
- ↑ Cobley, Christopher J.; Klosin, Jerzy; Qin, Cheng; Whiteker, Gregory T. (2004). "Parallel Ligand Screening on Olefin Mixtures in Asymmetric Hydroformylation Reactions". Org. Lett. 6 (19): 3277–80. doi:10.1021/ol0487938. PMID 15355031.
 |
