Chemistry:Ketopantoic acid
From HandWiki
| |
| Names | |
|---|---|
| Other names
4-Hydroxy-3,3-dimethyl-2-oxobutanoic acid; 2-Dehydropantoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 2242422 | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H10O4 | |
| Molar mass | 146.142 g·mol−1 |
| Appearance | colorless or white |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
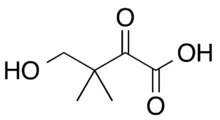
Ketopantoic acid is the organic compound with the formula HOCH2(CH3)2CC(O)CO2H. At physiological conditions, ketopantoic acid exists as its conjugate base, ketopantoate (HOCH2(CH3)2CC(O)CO2−).
Biosynthetic context
Its biosynthesis proceeds from ketoisovalerate by hydroxymethylation:
- (CH3)2CHC(O)CO2− + CH2O → HOCH2(CH3)2CC(O)CO2−
This conversion is catalyzed by ketopantoate hydroxymethyltransferase, which gives ketopantoate.
Ketopantoate is substrate for 2-dehydropantoate 2-reductase,[1] which produces pantoate, a precursor to pantothenic acid, a common prosthetic group.[2]
References
- ↑ Matak-Vinković, Dijana; Vinković, Mladen; Saldanha, S. Adrian; Ashurst, Jennifer L.; von Delft, Frank; Inoue, Tsuyoshi; Miguel, Ricardo Nunez; Smith, Alison G. et al. (2001). "Crystal Structure of Escherichia coli Ketopantoate Reductase at 1.7 Å Resolution and Insight into the Enzyme Mechanism". Biochemistry 40 (48): 14493–14500. doi:10.1021/bi011020w. PMID 11724562.
- ↑ Begley, Tadhg P.; Kinsland, Cynthia; Strauss, Erick (2001). "The Biosynthesis of Coenzyme a in Bacteria". Cofactor Biosynthesis. Vitamins & Hormones. 61. pp. 157–171. doi:10.1016/S0083-6729(01)61005-7. ISBN 9780127098616.
 |

