Chemistry:Kopsanone
From HandWiki
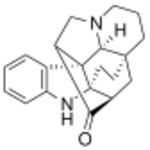
| |
| Names | |
|---|---|
| IUPAC name
5,14-diazaheptacyclo[12.5.3.01,13.04,12.04,18.06,11.012,16]docosa-6,8,10-trien-17-one
| |
| Other names
Kopsan-22-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H22N2O | |
| Molar mass | 306.409 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Kopsanone is an alkaloid isolated from Aspidosperma.[1]
References
- ↑ Mitaine, AC; Mesbah, K; Richard, B; Petermann, C; Arrazola, S; Moretti, C; Zèches-Hanrot, M; Men-Olivier, LL (1996). "Alkaloids from Aspidosperma species from Bolivia". Planta Med. 62 (5): 458–61. doi:10.1055/s-2006-957939. PMID 17252481.
Extra reading
- Klein-Júnior, Lc; Bannwart, G; Kato, L; Oliveira, Cma; Gontijo, B; Silva, Cc; Ferreira, Hd; Vander Heyden, Y et al. (25 November 2015). "Kopsanone and N(4)-oxide-kopsanone: two β-carbolinic indole alkaloids with monoamine oxidase A inhibitory activity". Planta Medica 81 (16): s–0035–1565743. doi:10.1055/s-0035-1565743.
- Craven, B. M. (1 October 1969). "The crystal structure and absolute configuration of the N ( b )-methiodide of (−)-kopsanone". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 25 (10): 2131–2139. doi:10.1107/S0567740869005243.
- Leng, Lingying; Zhou, Xiaohan; Liao, Qi; Wang, Falu; Song, Hao; Zhang, Dan; Liu, Xiao-Yu; Qin, Yong (20 March 2017). "Asymmetric Total Syntheses of Kopsia Indole Alkaloids". Angewandte Chemie International Edition 56 (13): 3703–3707. doi:10.1002/anie.201700831.
- Layne, Tanya H.; Roach, Joy S.; Tinto, Winston F. (January 2015). "Review of β-carboline Alkaloids from the Genus Aspidosperma". Natural Product Communications 10 (1): 1934578X1501000. doi:10.1177/1934578X1501000139.

 |
