Chemistry:Krische allylation
This article relies too much on references to primary sources. (August 2021) (Learn how and when to remove this template message) |
The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol.[1][2] The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols (precluding alcohol to aldehyde oxidation).[1][3]
Background
Enantioselective carbonyl allylations are frequently applied to the synthesis of polyketide natural products.[3] In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor.[4][5] Subsequently, other chiral allylmetal reagents were developed by Kumada, Roush, Brown, Leighton, and others.[6][7][8][9][10][11] These methods utilize preformed allyl metal reagents and generate stoichiometric quantities of metal byproducts.
In 1991, Yamamoto disclosed the first catalytic enantioselective method for carbonyl allylation, which employed a chiral boron Lewis acid-catalyst in combination with allyltrimethylsilane.[12] Numerous catalytic enantioselective methods for carbonyl allylation followed, including work by Umani-Ronchi[13] and Keck.[14] While these methods had a significant impact, they do not circumvent the use of preformed allylmetal reagents. Catalytic variants of the Nozaki-Hiyama-Kishi reaction represent an alternative method for asymmetric carbonyl allylation, but stoichiometric metallic reductants are required.[15]
Whereas the allylmetal reagents used in these first-generation technologies are often difficult to prepare and handle, the Krische allylation exploits highly tractable allylic acetates. Additionally, the Krische allylation avoids the use of preformed allyl metal reagents or metallic reductants and chiral auxiliaries, significantly reducing waste generation.
Reaction features
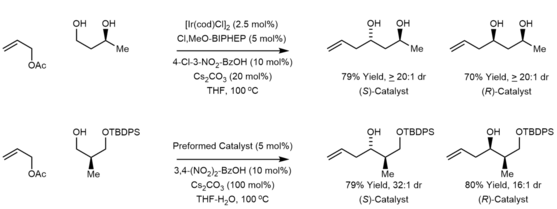
The Krische allylation involves “transfer hydrogenative” carbon-carbon bond formations.[16] In a series of papers published in the early 2000s, Krische and coworkers demonstrated that allenes, dienes, and allyl acetates could be converted to transient allylmetal nucleophiles via hydrogenation, transfer hydrogenation or hydrogen auto-transfer.[17] This strategy for enantioselective carbonyl allylation avoids preformed organometallic reagents or metallic reductants. A remarkable feature of these reactions is the ability to conduct carbonyl allylation from the alcohol oxidation state. Due to a kinetic preference for primary alcohol dehydrogenation, diols containing both primary and secondary alcohols undergo site-selective carbonyl allylation at the primary alcohol without the need for protecting groups.[18] Additionally, by using alcohol reactants, the use of chiral α-stereogenic aldehydes, which are prone to racemization, can be avoided.[19]
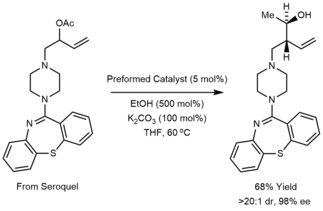
The excellent functional group compatibility of the Krische allylation combined with the tractability of the allyl acetate pronucleophiles enables the use of allyl donors bearing highly complex nitrogen-rich substituents.[20]
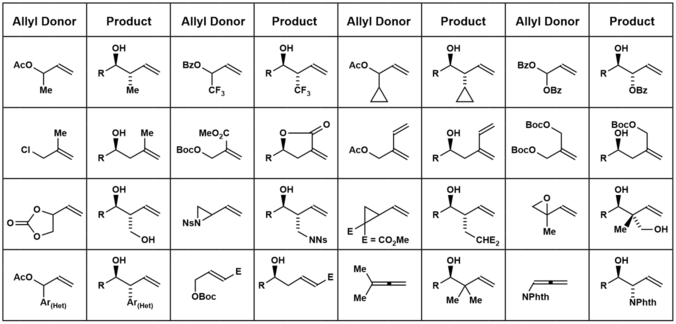
The figure below shows some of the different allyl donors that have been used in the Krische allylation. These methods are summarized in the review literature.[16][17]
Mechanism
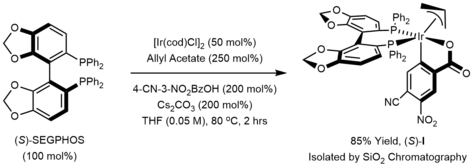
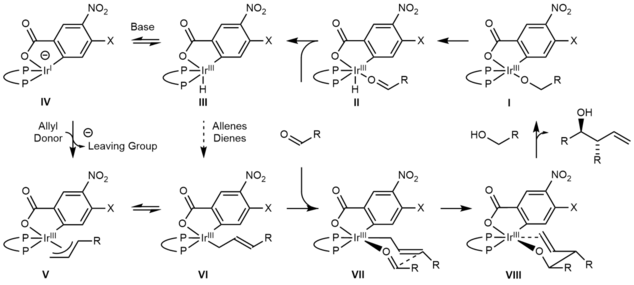
The active catalyst in the Krische allylation is a cyclometallated π-allyliridium C,O-benzoate complex. This complex can be generated in situ or can be isolated via precipitation or conventional chromatography on silica gel.
The mechanism of the Krische allylation has been corroborated by DFT calculations.[21] Entry into the catalytic cycle involves protonation of the cyclometallated π-allyliridium precatalyst to generate the iridium alkoxide I. β-Hydride elimination of alkoxide I generates the aldehyde, which dissociates to form the iridium hydride III. Deprotonation of the iridium hydride III provides an anionic iridium(I) species IV, which upon oxidative addition to the allyl donor forms the π-allyliridium complex V. Association of the aldehyde to the σ-allyliridium species VI triggers carbonyl addition by way of the six-centered transition structure VII to form the homoallylic alkoxide VIII. The homoallylic alkoxide VIII is stable with respect to beta-hydride elimination due to coordination of the double bond with the metal. Exchange with the primary alcohol reactant regenerates the iridium alkoxide I and releases the reaction product.
Applications in synthesis
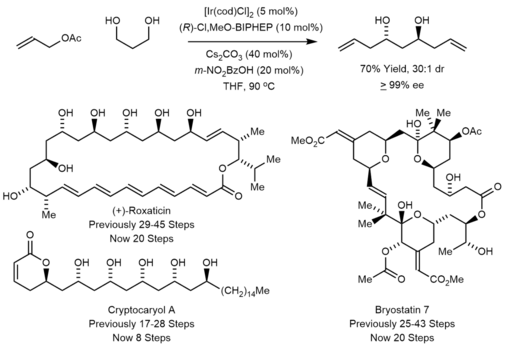
Iridium-catalyzed transfer-hydrogenative carbonyl allylation method has been applied to the synthesis of polyketide natural products.[3] Some examples are shown below. In every case, the target compound was prepared in significantly fewer steps than was previously achieved. For example, total syntheses of roxaticin, bryostatin and cryptocaryol were accomplished via double Krische allylation of 1,3-propane diol.[22][23][24] This method was also used in the synthesis of mandelalide A.[25]
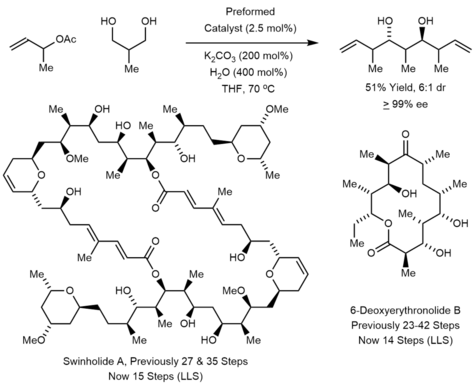
The Krische bisallylation has been applied to the synthesis of psymberin in 17 LLS and 32 total steps.[26] Through the use of the Krische allylation, this synthesis was accomplished via a much shorter route than previous syntheses. The Krische allylation to his synthesis of callyspongiolide using the chiral SEGPHOS catalyst complex.[27] ] In 2018, Harran also prepared callyspongiolide using the Krische allylation as a convergent method for fragment union.[28] Double crotylation was used by Krische to prepare 6-deoxyerythronolide B and swinholide A.[29][30]
Related articles
References
- ↑ 1.0 1.1 Kim, In Su; Ngai, Ming-Yu; Krische, Michael J. (2008-11-05). "Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level via Transfer Hydrogenative Coupling of Allyl Acetate: Departure from Chirally Modified Allyl Metal Reagents in Carbonyl Addition" (in en). Journal of the American Chemical Society 130 (44): 14891–14899. doi:10.1021/ja805722e. ISSN 0002-7863. PMID 18841896.
- ↑ Strategies and Tactics in Organic Synthesis, Volume 10 Michael Harmata Ed.
- ↑ 3.0 3.1 3.2 Feng, Jiajie; Kasun, Zachary A.; Krische, Michael J. (2016-05-04). "Enantioselective Alcohol C–H Functionalization for Polyketide Construction: Unlocking Redox-Economy and Site-Selectivity for Ideal Chemical Synthesis" (in en). Journal of the American Chemical Society 138 (17): 5467–5478. doi:10.1021/jacs.6b02019. ISSN 0002-7863. PMID 27113543.
- ↑ Herold, Thomas; Hoffmann, Reinhard W. (October 1978). "Enantioselective Synthesis of Homoallyl Alcoholsvia Chiral Allylboronic Esters" (in en). Angewandte Chemie International Edition in English 17 (10): 768–769. doi:10.1002/anie.197807682. ISSN 0570-0833. https://onlinelibrary.wiley.com/doi/10.1002/anie.197807682.
- ↑ Hoffmann, Reinhard W.; Herold, Thomas (January 1981). "Stereoselektive Synthese von Alkoholen, VII1) Optisch aktive Homoallylalkohole durch Addition chiraler Boronsäureester an Aldehyde" (in de). Chemische Berichte 114 (1): 375–383. doi:10.1002/cber.19811140139. https://onlinelibrary.wiley.com/doi/10.1002/cber.19811140139.
- ↑ Hayashi, Tamio; Konishi, Mitsuo; Kumada, Makoto (September 1982). "Optically active allylsilanes. 2. High stereoselectivity in asymmetric reaction with aldehydes producing homoallylic alcohols" (in en). Journal of the American Chemical Society 104 (18): 4963–4965. doi:10.1021/ja00382a046. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00382a046.
- ↑ Brown, Herbert C.; Jadhav, Prabhakar K. (April 1983). "Asymmetric carbon-carbon bond formation via .beta.-allyldiisopinocampheylborane. Simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purities" (in en). Journal of the American Chemical Society 105 (7): 2092–2093. doi:10.1021/ja00345a085. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00345a085.
- ↑ Roush, William R.; Walts, Alan E.; Hoong, Lee K. (December 1985). "Diastereo- and enantioselective aldehyde addition reactions of 2-allyl-1,3,2-dioxaborolane-4,5-dicarboxylic esters, a useful class of tartrate ester modified allylboronates" (in en). Journal of the American Chemical Society 107 (26): 8186–8190. doi:10.1021/ja00312a062. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00312a062.
- ↑ Kinnaird, James W. A.; Ng, Pui Yee; Kubota, Katsumi; Wang, Xiaolun; Leighton, James L. (2002-07-01). "Strained Silacycles in Organic Synthesis: A New Reagent for the Enantioselective Allylation of Aldehydes" (in en). Journal of the American Chemical Society 124 (27): 7920–7921. doi:10.1021/ja0264908. ISSN 0002-7863. PMID 12095334. https://pubs.acs.org/doi/10.1021/ja0264908.
- ↑ Short, Robert P.; Masamune, Satoru (March 1989). "Asymmetric allylboration with B-allyl-2-(trimethylsilyl)borolane" (in en). Journal of the American Chemical Society 111 (5): 1892–1894. doi:10.1021/ja00187a061. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00187a061.
- ↑ Corey, E. J.; Yu, Chan Mo; Kim, Sung Soo (July 1989). "A practical and efficient method for enantioselective allylation of aldehydes" (in en). Journal of the American Chemical Society 111 (14): 5495–5496. doi:10.1021/ja00196a082. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00196a082.
- ↑ Furuta, Kyoji; Mouri, Makoto; Yamamoto, Hisashi (1991). "Chiral (Acyloxy)borane Catalyzed Asymmetric Allylation of Aldehydes" (in en). Synlett 1991 (8): 561–562. doi:10.1055/s-1991-20797. ISSN 0936-5214. http://www.thieme-connect.de/DOI/DOI?10.1055/s-1991-20797.
- ↑ Costa, Anna Luisa; Piazza, Maria Giulia; Tagliavini, Emilio; Trombini, Claudio; Umani-Ronchi, Achille (July 1993). "Catalytic asymmetric synthesis of homoallylic alcohols" (in en). Journal of the American Chemical Society 115 (15): 7001–7002. doi:10.1021/ja00068a079. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00068a079.
- ↑ Keck, Gary E.; Tarbet, Kenneth H.; Geraci, Leo S. (September 1993). "Catalytic asymmetric allylation of aldehydes" (in en). Journal of the American Chemical Society 115 (18): 8467–8468. doi:10.1021/ja00071a074. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00071a074.
- ↑ Hargaden, Gráinne C.; Guiry, Patrick J. (2007-11-05). "The Development of the Asymmetric Nozaki–Hiyama–Kishi Reaction" (in en). Advanced Synthesis & Catalysis 349 (16): 2407–2424. doi:10.1002/adsc.200700324. https://onlinelibrary.wiley.com/doi/10.1002/adsc.200700324.
- ↑ 16.0 16.1 Santana, Catherine Gazolla; Krische, Michael J. (2021-05-07). "From Hydrogenation to Transfer Hydrogenation to Hydrogen Auto-Transfer in Enantioselective Metal-Catalyzed Carbonyl Reductive Coupling: Past, Present, and Future" (in en). ACS Catalysis 11 (9): 5572–5585. doi:10.1021/acscatal.1c01109. ISSN 2155-5435. PMID 34306816.
- ↑ 17.0 17.1 Kim, Seung Wook; Zhang, Wandi; Krische, Michael J. (2017-09-19). "Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier" (in en). Accounts of Chemical Research 50 (9): 2371–2380. doi:10.1021/acs.accounts.7b00308. ISSN 0001-4842. PMID 28792731.
- ↑ Dechert-Schmitt, Anne-Marie R.; Schmitt, Daniel C.; Krische, Michael J. (2013-03-11). "Protecting-Group-Free Diastereoselective CC Coupling of 1,3-Glycols and Allyl Acetate through Site-Selective Primary Alcohol Dehydrogenation" (in en). Angewandte Chemie International Edition 52 (11): 3195–3198. doi:10.1002/anie.201209863. PMID 23364927.
- ↑ Schmitt, Daniel C.; Dechert-Schmitt, Anne-Marie R.; Krische, Michael J. (2012-12-21). "Iridium-Catalyzed Allylation of Chiral β-Stereogenic Alcohols: Bypassing Discrete Formation of Epimerizable Aldehydes" (in en). Organic Letters 14 (24): 6302–6305. doi:10.1021/ol3030692. ISSN 1523-7060. PMID 23231774.
- ↑ Meyer, Cole C.; Stafford, Nicholas P.; Cheng, Melinda J.; Krische, Michael J. (2021-05-03). "Ethanol: Unlocking an Abundant Renewable C 2 ‐Feedstock for Catalytic Enantioselective C−C Coupling" (in en). Angewandte Chemie International Edition 60 (19): 10542–10546. doi:10.1002/anie.202102694. ISSN 1433-7851. PMID 33689214.
- ↑ Kim, Seung Wook; Meyer, Cole C.; Mai, Binh Khanh; Liu, Peng; Krische, Michael J. (2019-10-04). "Inversion of Enantioselectivity in Allene Gas versus Allyl Acetate Reductive Aldehyde Allylation Guided by Metal-Centered Stereogenicity: An Experimental and Computational Study" (in en). ACS Catalysis 9 (10): 9158–9163. doi:10.1021/acscatal.9b03695. ISSN 2155-5435. PMID 31857913.
- ↑ Han, Soo Bong; Hassan, Abbas; Kim, In Su; Krische, Michael J. (2010-11-10). "Total Synthesis of (+)-Roxaticin via C−C Bond Forming Transfer Hydrogenation: A Departure from Stoichiometric Chiral Reagents, Auxiliaries, and Premetalated Nucleophiles in Polyketide Construction" (in en). Journal of the American Chemical Society 132 (44): 15559–15561. doi:10.1021/ja1082798. ISSN 0002-7863. PMID 20961111.
- ↑ Lu, Yu; Woo, Sang Kook; Krische, Michael J. (2011-09-07). "Total Synthesis of Bryostatin 7 via C–C Bond-Forming Hydrogenation" (in en). Journal of the American Chemical Society 133 (35): 13876–13879. doi:10.1021/ja205673e. ISSN 0002-7863. PMID 21780806.
- ↑ Perez, Felix; Waldeck, Andrew R.; Krische, Michael J. (2016-04-11). "Total Synthesis of Cryptocaryol A by Enantioselective Iridium-Catalyzed Alcohol C−H Allylation" (in en). Angewandte Chemie International Edition 55 (16): 5049–5052. doi:10.1002/anie.201600591. PMID 27079820.
- ↑ Willwacher, Jens; Fürstner, Alois (2014-04-14). "Catalysis-Based Total Synthesis of Putative Mandelalide A" (in en). Angewandte Chemie International Edition 53 (16): 4217–4221. doi:10.1002/anie.201400605. PMID 24623640. http://doi.wiley.com/10.1002/anie.201400605.
- ↑ Feng, Yu; Jiang, Xin; De Brabander, Jef K. (2012-10-17). "Studies toward the Unique Pederin Family Member Psymberin: Full Structure Elucidation, Two Alternative Total Syntheses, and Analogs" (in en). Journal of the American Chemical Society 134 (41): 17083–17093. doi:10.1021/ja3057612. ISSN 0002-7863. PMID 23004238.
- ↑ Zhou, Jingjing; Gao, Bowen; Xu, Zhengshuang; Ye, Tao (2016-06-08). "Total Synthesis and Stereochemical Assignment of Callyspongiolide" (in en). Journal of the American Chemical Society 138 (22): 6948–6951. doi:10.1021/jacs.6b03533. ISSN 0002-7863. PMID 27227371. https://pubs.acs.org/doi/10.1021/jacs.6b03533.
- ↑ Manoni, Francesco; Rumo, Corentin; Li, Liubo; Harran, Patrick G. (2018-01-31). "Unconventional Fragment Usage Enables a Concise Total Synthesis of (−)-Callyspongiolide" (in en). Journal of the American Chemical Society 140 (4): 1280–1284. doi:10.1021/jacs.7b13591. ISSN 0002-7863. PMID 29332397. https://pubs.acs.org/doi/10.1021/jacs.7b13591.
- ↑ Gao, Xin; Woo, Sang Kook; Krische, Michael J. (2013-03-20). "Total Synthesis of 6-Deoxyerythronolide B via C–C Bond-Forming Transfer Hydrogenation" (in en). Journal of the American Chemical Society 135 (11): 4223–4226. doi:10.1021/ja4008722. ISSN 0002-7863. PMID 23464668.
- ↑ Shin, Inji; Hong, Suckchang; Krische, Michael J. (2016-11-02). "Total Synthesis of Swinholide A: An Exposition in Hydrogen-Mediated C–C Bond Formation" (in en). Journal of the American Chemical Society 138 (43): 14246–14249. doi:10.1021/jacs.6b10645. ISSN 0002-7863. PMID 27779393.
External links
 |