Chemistry:L-371,257
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
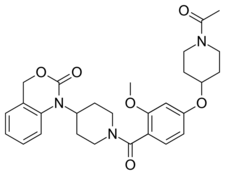
| Formula | C28H33N3O6 |
| Molar mass | 507.587 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
L-371,257 is a compound used in scientific research which acts as a selective antagonist of the oxytocin receptor with over 800x selectivity over the related vasopressin receptors.[1] It was one of the first non-peptide oxytocin antagonists developed,[2][3][4][5] and has good oral bioavailability, but poor penetration of the blood–brain barrier, which gives it good peripheral selectivity with few central side effects.[6] Potential applications are likely to be in the treatment of premature labour.[7]
See also
References
- ↑ "1-(1-[4-[(N-acetyl-4-piperidinyl)oxy]-2-methoxybenzoyl]piperidin-4- yl)-4H-3,1-benzoxazin-2(1H)-one (L-371,257): a new, orally bioavailable, non-peptide oxytocin antagonist". Journal of Medicinal Chemistry 38 (23): 4634–6. November 1995. doi:10.1021/jm00023a002. PMID 7473590.
- ↑ "Development of orally active oxytocin antagonists: studies on 1-(1-[4-[1-(2-methyl-1-oxidopyridin-3-ylmethyl)piperidin-4-yloxy]-2- methoxybenzoyl]piperidin-4-yl)-1,4-dihydrobenz[d][1,3]oxazin-2-one (L-372,662) and related pyridines". Journal of Medicinal Chemistry 41 (12): 2146–63. June 1998. doi:10.1021/jm9800797. PMID 9622556.
- ↑ "Nonpeptide oxytocin antagonists: potent, orally bioavailable analogs of L-371,257 containing a 1-R-(pyridyl)ethyl ether terminus". Bioorganic & Medicinal Chemistry Letters 8 (21): 3081–6. November 1998. doi:10.1016/S0960-894X(98)00568-X. PMID 9873680.
- ↑ "Nonpeptide oxytocin antagonists: analogs of L-371,257 with improved potency". Bioorganic & Medicinal Chemistry Letters 9 (9): 1311–6. May 1999. doi:10.1016/S0960-894X(99)00181-X. PMID 10340620.
- ↑ "Identification of potent and selective oxytocin antagonists. Part 1: indole and benzofuran derivatives". Bioorganic & Medicinal Chemistry Letters 12 (10): 1399–404. May 2002. doi:10.1016/S0960-894X(02)00159-2. PMID 11992786.
- ↑ "Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications". Psychopharmacology 185 (2): 218–25. April 2006. doi:10.1007/s00213-005-0293-z. PMID 16418825.
- ↑ "A Gly/Ala switch contributes to high affinity binding of benzoxazinone-based non-peptide oxytocin receptor antagonists". FEBS Letters 579 (2): 349–56. January 2005. doi:10.1016/j.febslet.2004.10.108. PMID 15642343.
 |

