Chemistry:Lamellarin D
| |
| Names | |
|---|---|
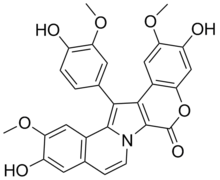
| Preferred IUPAC name
3,11-Dihydroxy-14-(4-hydroxy-3-methoxyphenyl)-2,12-dimethoxy-6H-[1]benzopyrano[4′,3′:4,5]pyrrolo[2,1-a]isoquinolin-6-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H21NO8 | |
| Molar mass | 499.475 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lamellarins are a group of pyrrole alkaloids first isolated in 1985 from the marine mollusk Lamellaria in the waters of Palau. Over 70 lamellarins and similar compounds were subsequently isolated. Other similar compounds include ningalins, lukianols, polycitones, and storniamides.[1]
Biological activity
These compounds have shown a wide variety of biological activity, including reversal of multidrug resistance, HIV-1 integrase inhibition, and antibiotic activity. Lamellarin D, for example, displays strong cytotoxic activity against tumor cell lines, and is a potent topoisomerase I inhibitor.[2]
Structure
The lamellarins all contain a central pyrrole ring, substituted at the 3 and 4 positions by polyhydroxy- or methoxyphenyls. They are divided into two groups, depending on whether the pyrrole ring is fused or unfused.[3]
Synthesis
The lamellarins have been synthesized by a number of groups, including Isibashi, Steglich, Ruchirawat, Banwell, Alvarez, Gupton, Boger, and Handy.[4]
Steglich synthesis of lamellarin G trimethyl ether
The Steglich synthesis features an oxidative coupling of two benzylic carbons, as well as a Paal-Knorr pyrrole synthesis.[5][6]
Banwell synthesis of lamellarin K
The Banwell group’s synthesis of lamellarin K includes an intramolecular azomethine ylide cyclization.[7]
Gallery
See also
References
- ↑ Bailly, Christian; Newman, David J. (2015). written at Pierre Fabre Research Institute. "Download Limit Exceeded" (in en) (PDF). Marine Drugs (2015-02-19) 13 (3): 1105–1123. doi:10.3390/md13031105. ISSN 1660-3397. PMID 25706633.
- ↑ Reddy, Venkata M.; Rao, Rama M.; Rhodes, Denise; Hansen, Mark S.; Rubins, Kathleen; Bushman, Frederic D.; Venkateswarlu, Yenamandra; Faulkner, John D. (November 24, 1999). "Lamellarin r 20-Sulfate, an Inhibitor of HIV-1 Integrase Active against HIV Virus in Cell Culture". Journal of Medicinal Chemistry (Indian Institute of Chemical Technology) 42 (11): 1901–1907. doi:10.1021/jm9806650. ISSN 0022-2623. PMID 10354398. http://microb230.med.upenn.edu/assets/pdf/publications/Reddy,etal.JMC42.pdf. Retrieved 2022-08-11.
- ↑ Bailly, Christian (2004-07-04). "Lamellarins, from A to Z: a family of anticancer marine pyrrole alkaloids". Current Medicinal Chemistry. Anti-Cancer Agents 4 (4): 363–378. doi:10.2174/1568011043352939. ISSN 1568-0118. PMID 15281908. https://pubmed.ncbi.nlm.nih.gov/15281908/.
- ↑ Ruchirawat, Somsak; Mutarapat, Thumnoon; Sahakitpichan, Poolsak; Bhavakul, Vanida; Mahidol, Chulabhorn (1997-11-13). "The Syntheses of Lamellarins and Isoindolobenzazepine Alkaloids". International Conference on Biodiversity and Bioresources (Phuket, Thailand) 70 (11): 6. https://media.iupac.org/symposia/proceedings/phuket97/ruchirawat.pdf. Retrieved 2022-08-11.
- ↑ "Paal-Knorr Pyrrole Synthesis" (in en), Comprehensive Organic Name Reactions and Reagents (Hoboken, NJ, USA: John Wiley & Sons, Inc.): pp. conrr475, 2010-09-15, doi:10.1002/9780470638859.conrr475, ISBN 978-0-470-63885-9, https://onlinelibrary.wiley.com/doi/10.1002/9780470638859.conrr475, retrieved 2022-08-11[|permanent dead link|dead link}}]
- ↑ "Hantzsch Pyrrole Synthesis" (in en), Comprehensive Organic Name Reactions and Reagents (Hoboken, NJ, USA: John Wiley & Sons, Inc.): pp. conrr295, 2010-09-15, doi:10.1002/9780470638859.conrr295, ISBN 978-0-470-63885-9, https://onlinelibrary.wiley.com/doi/10.1002/9780470638859.conrr295, retrieved 2022-08-11[|permanent dead link|dead link}}]
- ↑ Padwa, Albert (2017-11-29). "Use of nitrogen and oxygen dipole ylides for alkaloid synthesis" (in en) (PDF). The Free Internet Journal for Organic Chemistry 5 (1): 23–49. 2018-02-06. doi:10.24820/ark.5550190.p010.416. https://www.arkat-usa.org/get-file/62477/. Retrieved 2022-08-11.
 |



