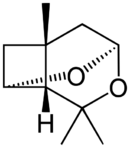
Chemistry:Lineatin
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1R,2S,5R,7S)-1,3,3-Trimethyl-4,6-dioxatricyclo[3.3.1.02,7]nonane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H16O2 | |
| Molar mass | 168.2 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lineatin is a pheromone produced by female striped ambrosia beetle, Trypodendron lineatum Olivier. These kinds of beetles are responsible for extensive damage of coniferous forest infestation in Europe and North America. Since lineatin can act as lures used for mass-trapping of T. lineatum, it is being studied to apply as a pest control reagent.
Structure
Lineatin was first isolated in 1977 by MacConnell.[1] The absolute configuration of the biologically active form was later determined as (+)-(1R,4S,5R,7R)-3,3,7-trimethyl-2,9- dioxatricyclo[3.3.1.04,7]nonane, whereas other enatinomers process no biological attraction activity.[2]
Total Synthesis
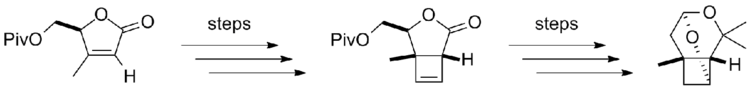
After the absolute structure was determined, lineatin quickly attracted considerable synthetic interests due to its natural occurrence, biological activity, and unique structural features. A few routes describing the total synthesis of lineatin was proposed with yields of 0.5–2%.[3][4][5][6] Recently, a new total synthesis route that adopted a photochemical [2 + 2] cycloaddition approach to construct diastereoselective cyclobutene and a regiocontrolled oxymercuration reaction was proposed. This route achieved in synthesizing highly pure (+)-lineatin (> 99.5% ee) through 14 steps and resulted in 14% overall yield from a homochiral 2(5H)-furanone. (Figure 1 showed the basic outline of this approach).[7]
Biosynthesis
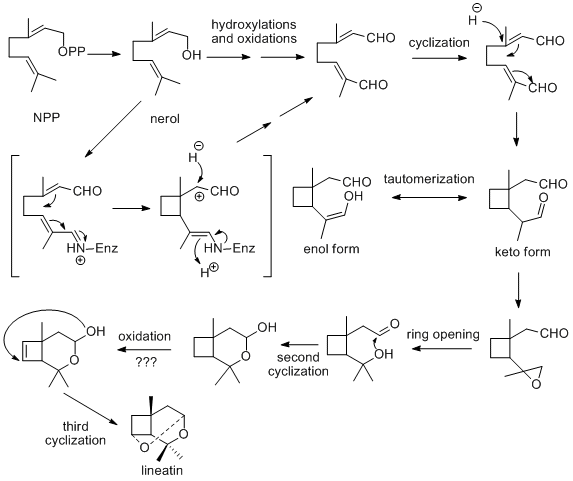
Lineatin is a monoterprene with unique tricyclic acetal structure. Most of the studies regarding lineatin were focused on the total synthesis; little attentions were put on its biosynthesis. It is suggested that lineatin is derived through oxidation and cyclization of a monoterponid precursor, but no experimental has been done on proving this route.[8] Based on its partial structure similarity to iridoid class of terprenoids, here, a possible biosynthesis pathway was proposed and outlined in figure 2.
References
- ↑ MacConnell, J. G.; Borden, J. H.; Silverstein, R. M.; Stokkink, E. (1977). "Isolation and tentative identification of lineatin, a pheromone from the frass ofTrypodendron lineatum (Coleoptera: Scolytidae)". J. Chem. Ecol. 3 (5): 549–561. doi:10.1007/BF00989076.
- ↑ Slessor, K. N.; Oehlschlager, A. C.; Johnston, B. D.; Pierce, H. D.; Grewel, S. K.; Wickremesinghe, L. K. G. (1980). "Lineatin: regioselective synthesis and resolution leading to the chiral pheromone of Trypodendron lineatum". J. Org. Chem. 45 (12): 2290. doi:10.1021/jo01300a005.
- ↑ Mori, K.; Sasaki, M. (1980). "Synthesis of racemic and optically active forms of lineatin, the unique tricyclic pheromone of trypodendron lineatum (olivier)". Tetrahedron 36 (15): 2197–2208. doi:10.1016/0040-4020(80)80112-8.
- ↑ Mori, K.; Uematsu, T.; Minobe, M.; Yanagi, K. (1983). "Synthesis and absolute configuration of both the enantiomers of lineatin The pheromone of trypodendron lineatum". Tetrahedron 39 (10): 1735–1743. doi:10.1016/S0040-4020(01)88680-4.
- ↑ Kandil, A. A.; Slessor, K. N. (1985). "A chiral synthesis of (+)-lineatin, the aggregation pheromone of Trypodendron lineatum (Olivier), from D-ribonolactone". J. Org. Chem. 50 (26): 5649–5655. doi:10.1021/jo00350a045.
- ↑ Mori, K.; Nagano, E. (1991). "Pheromone synthesis, CXXVII. A new synthesis of the enantiomers of grandisol and lineatin". Liebigs Ann. Chem. 1991 (4): 341–344. doi:10.1002/jlac.199119910159.
- ↑ Racamonde, M.; Alibes, R.; Figueredo, M.; Font, J.; de March, P. (2008). "Photochemical cycloaddition of mono-, 1,1-, and 1,2-disubstituted olefins to a chiral 2(5H)-furanone. Diastereoselective synthesis of (+)-lineatin". J. Org. Chem. 73 (15): 5944–5952. doi:10.1021/jo800970u. PMID 18605755.
- ↑ Vanderwel, D. (1991). PhD Thesis. Simon Fraser University.
 |
