Chemistry:Luminespib
| |
| Names | |
|---|---|
| Preferred IUPAC name
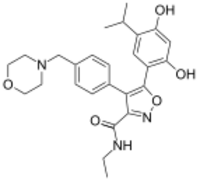
5-[2,4-Dihydroxy-5-(propan-2-yl)phenyl]-N-ethyl-4-{4-[(morpholin-4-yl)methyl]phenyl}-1,2-oxazole-3-carboxamide | |
| Other names
NVP-AUY-922; AUY922; VER-52296
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H31N3O5 | |
| Molar mass | 465.550 g·mol−1 |
| Appearance | Colorless solid[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Luminespib (INN,[2] previously known as NVP-AUY922) is an experimental drug candidate for the treatment of cancer. It was discovered through a collaboration between The Institute of Cancer Research and the pharmaceutical company Vernalis[3] and licensed to Novartis.[4] From 2011 to 2014 it was in Phase II clinical trials.[5][6] Chemically it is a resorcinylic isoxazole amide[6]
Luminespib is an inhibitor of heat shock protein 90 (Hsp90),[1] which is a chaperone protein that plays a role in the modification of a variety of proteins that have been implicated in oncogenesis. Luminespib has shown promising activity in preclinical testing against several different tumor types.[7][8][9][10]
A related compound, NVP-HSP990, was abandoned by Novartis in 2012 after it failed to show efficacy in an early clinical trial.[6]
See also
References
- ↑ 1.0 1.1 Brough, Paul A.; Aherne, Wynne; Barril, Xavier; Borgognoni, Jenifer; Boxall, Kathy; Cansfield, Julie E.; Cheung, Kwai-Ming J.; Collins, Ian et al. (2008). "4,5-Diarylisoxazole Hsp90 Chaperone Inhibitors: Potential Therapeutic Agents for the Treatment of Cancer". Journal of Medicinal Chemistry 51 (2): 196–218. doi:10.1021/jm701018h. PMID 18020435. https://figshare.com/articles/4_5_Diarylisoxazole_Hsp90_Chaperone_Inhibitors_Potential_Therapeutic_Agents_for_the_Treatment_of_Cancer/2961214.
- ↑ "WHO Drug Information. International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 70". World Health Organization. pp. 297–8. https://www.who.int/medicines/publications/druginformation/innlists/RL70.pdf.
- ↑ "Structure-based design of cancer therapeutics". The Institute of Cancer Research. http://www.icr.ac.uk/about_us/annual_research_report/9719.pdf.
- ↑ "AUY922". Vernalis. http://www.vernalis.com/development/oncology/auy922.
- ↑ "Small caps: Vernalis drug fillip". Financial Times. July 19, 2011. http://www.ft.com/intl/cms/s/0/715446aa-b219-11e0-9d80-00144feabdc0.html#axzz1TL06XsgO.
- ↑ 6.0 6.1 6.2 Sidera, K.; Patsavoudi, E. (Jan 2014). "HSP90 inhibitors: current development and potential in cancer therapy.". Recent Patents on Anti-Cancer Drug Discovery 9 (1): 1–20. doi:10.2174/15748928113089990031. PMID 23312026.
- ↑ Jensen, Michael; Schoepfer, Joseph; Radimerski, Thomas; Massey, Andrew; Guy, Chantale T; Brueggen, Josef; Quadt, Cornelia; Buckler, Alan et al. (2008). "NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models". Breast Cancer Research 10 (2): R33. doi:10.1186/bcr1996. PMID 18430202.
- ↑ Gaspar, N.; Sharp, S. Y.; Eccles, S. A.; Gowan, S.; Popov, S.; Jones, C.; Pearson, A.; Vassal, G. et al. (2010). "Mechanistic Evaluation of the Novel HSP90 Inhibitor NVP-AUY922 in Adult and Pediatric Glioblastoma". Molecular Cancer Therapeutics 9 (5): 1219–1233. doi:10.1158/1535-7163.MCT-09-0683. PMID 20457619.
- ↑ Okui, T; Shimo, T; Hassan, NM; Fukazawa, T; Kurio, N; Takaoka, M; Naomoto, Y; Sasaki, A (2011). "Antitumor effect of novel HSP90 inhibitor NVP-AUY922 against oral squamous cell carcinoma". Anticancer Research 31 (4): 1197–204. PMID 21508365.
- ↑ Eccles, S. A.; Massey, A.; Raynaud, F. I.; Sharp, S. Y.; Box, G.; Valenti, M.; Patterson, L.; De Haven Brandon, A. et al. (2008). "NVP-AUY922: A Novel Heat Shock Protein 90 Inhibitor Active against Xenograft Tumor Growth, Angiogenesis, and Metastasis". Cancer Research 68 (8): 2850–2860. doi:10.1158/0008-5472.CAN-07-5256. PMID 18413753.
 |

