Chemistry:Lythranidine
From HandWiki
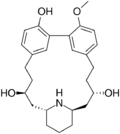
| |
| Names | |
|---|---|
| Systematic IUPAC name
(9S,11R,15R,17S)-23-Methoxy-25-azatetracyclo[18.3.1.12,6.111,15]hexacosa-1(24),2,4,6(26),20,22-hexaene-3,9,17-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| 1555440 | |
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C26H35NO4 | |
| Molar mass | 425.569 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lythranidine is a piperidine alkaloid that was first isolated from the plant Lythrum anceps.[1] It contains a 17-membered cyclophane ring.
Several laboratory syntheses have been reported.[2][3][4][5]
References
- ↑ Fujita, E; Fuji, K (1971). "Lythraceous alkaloids. Part IV. Structure and absolute configuration of lythranine, lythranidine, and lythramine". Journal of the Chemical Society C: Organic: 1651. doi:10.1039/J39710001651.
- ↑ Fuji, Kaoru; Ichikawa, Kohei; Fujita, Eiichi (1980). "Lythraceous alkaloids. Part 11. Total synthesis of (±)-lythranidine". J. Chem. Soc., Perkin Trans. 1: 1066–1069. doi:10.1039/P19800001066.
- ↑ Carruthers, William; Coggins, Peter; Weston, John B (1991). "Nitrone cycloaddition: An approach to the cyclophane alkaloid (±)-lythranidine". J. Chem. Soc., Perkin Trans. 1 (3): 611–616. doi:10.1039/P19910000611.
- ↑ Pinder, A. R (1992). "Azetidine, pyrrole, pyrrolidine, piperidine, and pyridine alkaloids". Natural Product Reports 9 (5): 491. doi:10.1039/NP9920900491.
- ↑ Gebauer, Konrad; Fürstner, Alois (2014). "Total Synthesis of the Biphenyl Alkaloid (−)-Lythranidine". Angewandte Chemie International Edition 53 (25): 6393–6396. doi:10.1002/anie.201402550. PMID 24821137. http://pubman.mpdl.mpg.de/pubman/item/escidoc:2034320:3/component/escidoc:2034319/anie_201402550_sm_miscellaneous_information.pdf.
 |
