Chemistry:M1G
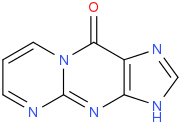
| |
| Names | |
|---|---|
| IUPAC name
Pyrimido[1,2-a]purin-10(3H)-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | C107643 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H5N5O | |
| Molar mass | 187.162 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
M1G (pyrimido[1,2-a]purin-10(3H)-one) is a heterocyclic compound which is a by-product of base excision repair (BER) of a specific type of DNA adduct called M1dG. The M1dG adduct in turn is formed by a condensation reaction between guanosine nucleotides in DNA and either malondialdehyde (propanedial) [1] or acrolein.[2] If not repaired, these adducts are mutagenic and carcinogenic.
Malondialdehyde is an end product of lipid peroxidation[2] while acrolein is a result of DNA peroxidation.[3]
M1dG is the major endogenous DNA adduct in humans. M1dG adducts have been detected in cell DNA in liver, leucocytes, pancreas and breast in concentrations of 1-120 per 108 nucleotides.[1] Detection and quantification of M1dG adducts in the body as measured by free M1G is a tool for detecting DNA damage that may lead to cancer. Free M1G is also biomarker for oxidative stress.[1]
References
- ↑ 1.0 1.1 1.2 Marnett LJ (1999). "Lipid peroxidation-DNA damage by malondialdehyde". Mutat. Res. 424 (1–2): 83–95. doi:10.1016/S0027-5107(99)00010-X. PMID 10064852. Bibcode: 1999MRFMM.424...83M.
- ↑ 2.0 2.1 "Reaction of Malonaldehyde with Nucleic Acid. I. Formation of Fluorescent Pyrimido[1,2-a]purin-10(3H)-one Nucleosides". Bulletin of the Chemical Society of Japan 56 (6): 1799–1802. 1983. doi:10.1246/bcsj.56.1799.
- ↑ "Metabolism in vitro and in vivo of the DNA base adduct, M1G". Chem. Res. Toxicol. 20 (3): 550–7. March 2007. doi:10.1021/tx600334x. PMID 17311424.
External links
- pyrimido(1,2-a)purin-10(3H)-one at the US National Library of Medicine Medical Subject Headings (MeSH)
- 3-(2'-deoxy-beta-D-erythro-pentofuranosyl)pyrimido(1,2-alpha)purin-10(3H)-one at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
