Chemistry:Maklamicin
| |
| Names | |
|---|---|
| IUPAC name
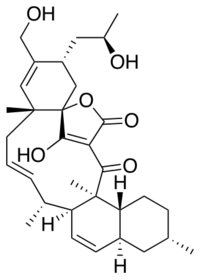
(1S,3R,6R,8E,10R,11S,14S,16S,19R,20S)-21-hydroxy-4-(hydroxymethyl)-3-[(2R)-2-hydroxypropyl]-6,10,16,20-tetramethyl-24-oxapentacyclo[20.2.1.01,6.011,20.014,19]pentacosa-4,8,12,21-tetraene-23,25-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C32H44O6 | |
| Molar mass | 524.698 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Maklamicin is a spirotetronate-class polyketide natural product. Isolated from Micromonospora sp. GMKU326 found in the root of Maklam phueak, it displays antibiotic activity against Gram-positive bacterial strains Micrococcus luteus, Bacillus subtilis, Bacillius cereus, Staphylococcus aureus, and Enterococcus faecalis.[1]
Biosynthesis
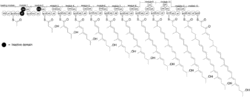
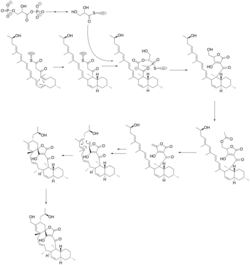
Maklamicin arises from a type I modular polyketide synthase (PKS) system. The structure of its polyketide chain extension has been shown to contain thirteen discrete PKS modules (a loading module and twelve extension modules).[2]
After passing through the extension domains the linear polyketide precursor to Maklamicin undergoes an intramolecular Diels-Alder reaction (IMDA) to form its trans-decalin motif. 1,3-bisphosphoglycerate is converted to glyceryl S-ACP and condenses onto the decalin-bearing polyketide intermediate to furnish an intermediate containing the premature tetronate moiety. This intermediate then acetylated and subsequently dehydrated to form the exocyclic olefin of the mature tetronate moiety. The intermediate, now containing both the tetronate and trans-decalin motifs of Maklamicin is then systematically reduced to afford a diene. A second IMDA then occurs between the tetronate and newly formed dienophile to yield the ultimate intermediate which is oxidized to afford Maklamicin.[3]
References
- ↑ Igarashi, Yasuhiro; Ogura, Hiromu; Furihata, Kazuo; Oku, Naoya; Indananda, Chantra; Thamchaipenet, Arinthip (2011). "Maklamicin, an Antibacterial Polyketide from an Endophytic Micromonosporasp". Journal of Natural Products 74 (4): 670–4. doi:10.1021/np100727h. PMID 21388191.
- ↑ Daduang, Ratama; Kitani, Shigeru; Hashimoto, Junko; Thamchaipenet, Arinthip; Igarashi, Yasuhiro; Shin-Ya, Kazuo; Ikeda, Haruo; Nihira, Takuya (2015). "Characterization of the biosynthetic gene cluster for maklamicin, a spirotetronate-class antibiotic of the endophytic Micromonospora sp. NBRC 110955". Microbiological Research 180: 30–9. doi:10.1016/j.micres.2015.07.003. PMID 26505309.
- ↑ Daduang, Ratama; Kitani, Shigeru; Hashimoto, Junko; Thamchaipenet, Arinthip; Igarashi, Yasuhiro; Shin-Ya, Kazuo; Ikeda, Haruo; Nihira, Takuya (2015). "Characterization of the biosynthetic gene cluster for maklamicin, a spirotetronate-class antibiotic of the endophytic Micromonospora sp. NBRC 110955". Microbiological Research 180: 30–9. doi:10.1016/j.micres.2015.07.003. PMID 26505309.
 |