Chemistry:Martinet dioxindole synthesis
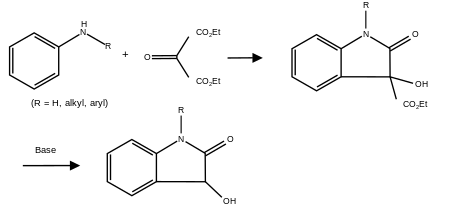
The Martinet dioxindole synthesis was first reported in 1913 by J. Martinet.[1] It is a chemical reaction in which a primary or secondary aniline or substituted aromatic amine is condensed with ethyl or methyl ester of mesoxalic acid to make a dioxindole in the absence of oxygen.[2]
Proposed mechanism
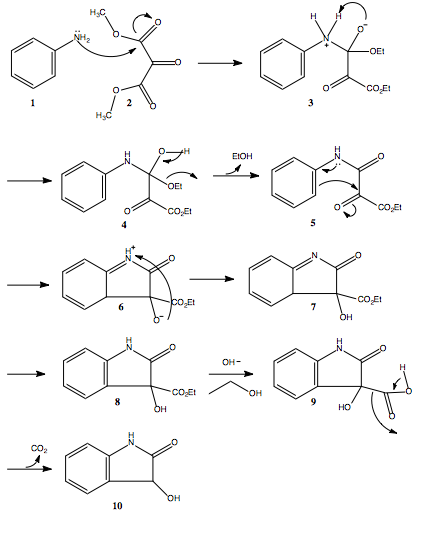
In the first step, the amino group on the aniline (1) attacks the carbonyl of the ethyl oxomalonate (2). A proton from the nitrogen is extracted by the oxygen and an alcohol group forms (3). The carbonyl re-forms to make a keto group and an ethanol molecule leaves (4). Next, a ring closing reaction occurs by the bond from the aromatic benzene ring attacking the partially positive carbonyl to form a five-member ring (5). After a proton transfer (6), an isomerization or a [1,3] hydride shift occurs and aromaticity is restored to the six-membered ring (7). In the presence of base, the ester is hydrolyzed, ethanol is lost (8) and a decarboxylation occurs (9). The resulting product is the desired dioxindole (10).[3]
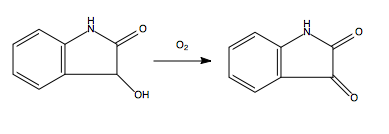
In the presence of oxygen, dioxindole converts to isatin through oxidation.[2]
Applications
The Martinet dioxindole synthesis is utilized in the preparation of oxindole derivatives. Oxindole derivatives found in natural products are gaining popularity in research because of their structural diversity. 3-substituted-3-hydroxy-2-oxindole is the central structure of a wide variety of biologically important compounds found in natural products. The 3-substituted-3-hydroxy-2-oxindole structure holds anti-oxidant, anti-cancer, anti-HIV, and neuroprotective properties. The utilization of this core structure for drug synthesis and the relevant cellular pathways involved are being extensively studied.[4] The enantio-selective addition of 3-substituted oxindole derivatives to different electrophiles gives access to chiral 3,3-disubstituted oxindole derivatives. The dioxindole is a strong nucleophile for the Michael addition of dioxindoles to nitroalkenes in order to obtain 3,3-disubstituted oxindole derivatives.[5]
Experimental examples
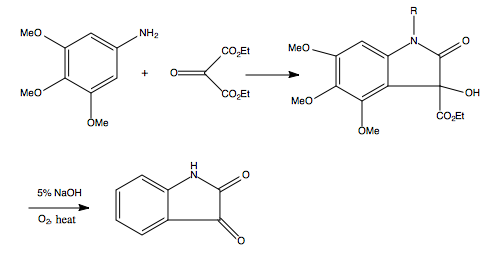
The Martinet dioxindole synthesis proceeds with an alkoxyaniline, 3,4,5-trimethoxyaniline, which reacts with an oxomalonic ester in glacial acetic acid to synthesize 2-carbethoxy-4,5,6-trimethoxyindoxyl, 2-carbethoxy-3,4,5,6-tetramethoxyindole and 4,5,6-trimethoxy-3-hydroxy-3-carbethoxyindole.[6]
Dioxindole
Dioxindole is a non-aromatic heterocyclic organic compound. It has a bicyclic structure consisting of a six-membered aromatic ring fused to a five-membered nitrogen containing ring. It is a hydroxy derivative of oxindole first prepared by reducing isatin with sodium amalgam in an alkaline solution.[2]
See also
References
- ↑ "Formation of derivatives of dioxindole from esters of mesoxalic acid and aromatic amines or amino quinolines". Compt. Rend. 156: 1625. 1913.
- ↑ 2.0 2.1 2.2 Sumpter, Ward C. (1945). "The Chemistry of Oxindole.". Chemical Reviews 37 (3): 443–479. doi:10.1021/cr60118a003. ISSN 0009-2665. PMID 21013427.
- ↑ Wang, Z. (2009) Comprehensive Organic Name Reactions and Reagents II. John Wiley and Sons, Inc.: Hoboken, NJ, p. 1839, ISBN:0471704504.
- ↑ Peddibhotla, S. (2009). "3-Substituted-3-hydroxy-2-oxindole, an Emerging New Scaffold for Drug Discovery with Potential Anti-Cancer and other Biological Activities". Current Bioactive Compounds 5 (1): 20–38. doi:10.2174/157340709787580900.
- ↑ "Dioxindole in asymmetric catalytic synthesis: direct access to 3-substituted 3-hydroxy-2-oxindoles via 1,4-additions to nitroalkenes". Chem. Commun. 48 (27): 3336–8. 2012. doi:10.1039/c2cc30198a. PMID 22362379.
- ↑ "Mescaline analogs. IV. substituted 4,5,6-trimethoxyindoles". The Journal of Organic Chemistry 20 (10): 1454–1457. 1955. doi:10.1021/jo01127a026. ISSN 0022-3263.
 |