Chemistry:Nitroalkene
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.[1]
Synthesis
Nitroalkenes are synthesized by various means, notable examples include:
- Nitroaldol reactions such as the Henry reaction:[1][2][3][4]
- Nitration of an alkene with nitryl iodide generated in-situ from silver nitrite and elemental iodine:[5]
center
- Direct nitration of alkenes with nitric oxide and an aluminum oxide catalyst in acidic conditions:[6]
center
- Direct nitration of alkenes with Clayfen (Iron(III) nitrate supported on Montmorillonite clay):[7]
center
- Dehydration of nitro-alcohols:[8]

Reactions
Nitroalkenes are useful intermediates for various chemical functionalities.
center
- Nitroalkene acting as an activated dienophile toward butadiene in a Diels-Alder cycloaddition:[1][10]
center
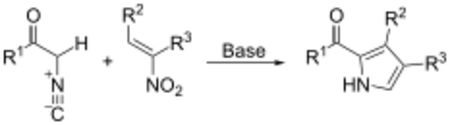
- The synthesis of pyrrole derivatives via the Barton–Zard reaction:[11]
- Pericyclic reaction of a nitroalkene yielding an indole:[12]
center
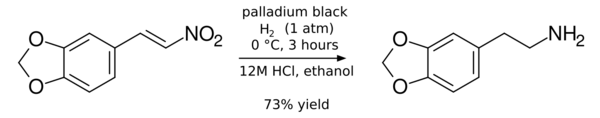
- Partial hydrogenation to an alkene baring a hydroxylamine functional group:[13]
center
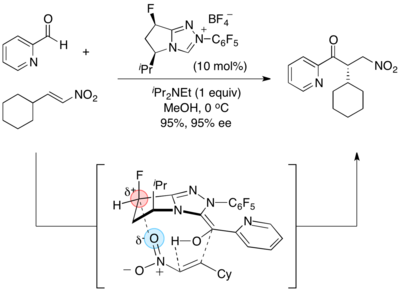
- Asymmetric Stetter reaction:[15]
Nitroalkynes
The related nitroalkynes are rather unstable, easily losing nitrogen dioxide radicals, rearranging to nitriles over −40 °C, or adding nucleophiles. Fewer than 20 had been synthesized before 2014. Nitration of metalloalkynes requires nearly-bare nitronium, i.e. nitronium tetrafluoroborate or nitric anhydride. In contrast, Tilden's reagent suffices to nitrosylate metalloalkynes; the products then oxidize to nitroalkenes in peroxyacids. Protected nitroalkene dehydroiodination occurs delicately in the gas phase.[16]
References
- ↑ 1.0 1.1 1.2 1.3 Furniss, Brian; Hannaford, Antony; Smith, Peter; Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed.. London: Longman Science & Technical. pp. 635, 768, 1035–1036, & 1121. ISBN 9780582462366. https://archive.org/details/TextbookOfPracticalOrganicChemistry5thEd.
- ↑ Ballini, Roberto; Castagnani, Roberto; Petrini, Marino (1992). "Chemoselective synthesis of functionalized conjugated nitroalkenes". The Journal of Organic Chemistry 57 (7): 2160–2162. doi:10.1021/jo00033a045.
- ↑ Worrall, David E. (1929). "Nitrostyrene". Org. Synth. 9: 66. doi:10.15227/orgsyn.009.0066.
- ↑ Chandrasekhar, S.; Shrinidhi, A. (2014). "Useful Extensions of the Henry Reaction: Expeditious Routes to Nitroalkanes and Nitroalkenes in Aqueous Media". Synthetic Communications 44 (20): 3008–3018. doi:10.1080/00397911.2014.926373. https://figshare.com/articles/journal_contribution/1053153.
- ↑ Waldman, Steve; Monte, Aaron, Monte; Bracey, Ann; Nichols, David (1996). "One-pot Claisen rearrangement/O-methylation/alkene isomerization in the synthesis of ortho-methoxylated phenylisopropylamines". Tetrahedron Letters 37 (44): 7889–7892. doi:10.1016/0040-4039(96)01807-2.
- ↑ Mukaiyama, T.; Hata E.; Yamada, T. (1995). "Convenient and Simple Preparation of Nitroolefins Nitration of Olefins with Nitric Oxide". Chemistry Letters 24 (7): 505–506. doi:10.1246/cl.1995.505.
- ↑ Varma, Rajender; Naicker, Kannan; Liesen, Per (1998). "Selective nitration of styrenes with clayfen and clayan: A solvent-free synthesis of β-nitrostyrenes". Tetrahedron Letters 39 (23): 3977–3980. doi:10.1016/S0040-4039(98)00740-0.
- ↑ Ranganathan, Darshan; Rao, Bhushan; Ranganathan, Subramania; Mehrotra, Ashok; Iyengar, Radha (1980). "Nitroethylene: a stable, clean, and reactive agent for organic synthesis". The Journal of Organic Chemistry 45 (7): 1185–1189. doi:10.1021/jo01295a003.
- ↑ Jubert, Carole; Knochel, Paul (1992). "Preparation of polyfunctional nitro olefins and nitroalkanes using the copper-zinc reagents RCu(CN)ZnI". The Journal of Organic Chemistry 57 (20): 5431–5438. doi:10.1021/jo00046a027.
- ↑ Noboru Ono; Hideyoshi Miyake; Akio Kamimura; Aritsune, Kaji (1987). "Regioselective Diels–Alder reactions. The nitro group as a regiochemical control element". Perkin Transactions 1: 1929–1935. doi:10.1039/P19870001929.
- ↑ Jie Jack Li (2013). Heterocyclic Chemistry in Drug Discovery. New York: Wiley. ISBN 9781118354421. https://books.google.com/books?id=p_fM6CuK6xcC. pp.43-4
- ↑ Novellino, Luisa; d'Ischia, Marco; Prota, Giuseppe (1999). "Expedient Synthesis of 5,6-Dihydroxyindole and Derivatives via an Improved Zn(II)-Assisted 2,β-Dinitrostyrene Approach". Synthesis 1999 (5): 793–796. doi:10.1055/s-1999-3469.
- ↑ 13.0 13.1 Masahiko Kohno; Shigehiro Sasao; Shun-Ichi Murahashi (1990). "Synthesis of Phenethylamines by Hydrogenation of β-Nitrostyrenes". Bulletin of the Chemical Society of Japan 63 (4): 1252–1254. doi:10.1246/bcsj.63.1252.
- ↑ Koch, Werner; Reichert, Benno (1935). "Über die katalytische Hydrierung substituierter ω-Nitrostyrole". Archiv der Pharmazie 273 (18–20): 265–274. doi:10.1002/ardp.19352731802.
- ↑ DiRocco, D. A.; Oberg, K. M.; Dalton, D. M.; Rovis, T. (2009). "Catalytic Asymmetric Intermolecular Stetter Reaction of Heterocyclic Aldehydes with Nitroalkenes: Backbone Fluorination Improves Selectivity". Journal of the American Chemical Society 131 (31): 10872–10874. doi:10.1021/ja904375q. PMID 19722669.
- ↑ Widler, G. Kenneth; Pagoria, Philip F.; Vollhardt, K. Peter C. (2014). "Nitroalkynes: A Unique Class of Energetic Materials". Synthesis (New York, NY: Thieme). https://www.researchgate.net/profile/Philip-Pagoria/publication/273040611_Nitroalkynes_A_Unique_Class_of_Energetic_Materials/links/587420e108ae329d621d3995/Nitroalkynes-A-Unique-Class-of-Energetic-Materials.pdf.
 |

