Chemistry:Metatartaric acid
From HandWiki
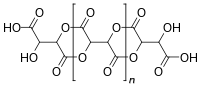 Idealized chemical structure of metartaric acid
| |
| Names | |
|---|---|
| Other names
E353
| |
| Identifiers | |
| |
| Properties | |
| (C4H4O5)n | |
| Molar mass | Variable |
| Appearance | Off-white solid[1] |
| Freely soluble[1] | |
| Solubility in ethanol | Soluble[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Metatartaric acid is a food additive. Chemically, it is a polymeric lactone of variable composition and different molecular weights obtained through a dehydration reaction by heating tartaric acid.[2]
Uses
As a food additive, it has the E number E353 and is classified as an acidity regulator.[1] It is added to wine to prevent the precipitation of potassium hydrogen tartrate and calcium tartrate.[3]
References
- ↑ 1.0 1.1 1.2 1.3 "Re-evaluation of metatartaric acid (E 353) as a food additive". EFSA Journal 18 (3): e06031. March 2020. doi:10.2903/j.efsa.2020.6031. PMID 32874249.
- ↑ "Cold Stabilization". Australian Wine Research Institute. https://www.awri.com.au/industry_support/winemaking_resources/storage-and-packaging/pre-packaging-preparation/cold-stabilisation/.
- ↑ "3.3.7 Treatment with Metatartaric Acid". International Code of Oenological Practices. https://www.oiv.int/public/medias/3504/e-code-ii-337.pdf.
 |

