Chemistry:Methanetellurol
From HandWiki
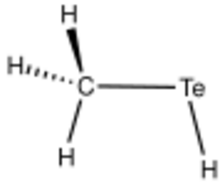
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| CH 3TeH | |
| Molar mass | 143.64 g·mol−1 |
| Appearance | colorless gas |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methanetellurol is the organotellurium compound with the formula CH
3TeH. It is the simplest organotellurium compound that has been purified in bulk. It is classified as a tellurol. A colorless gas, it decomposes to Te and methane near room temperature. It is prepared by reduction of dimethyl ditelluride using Na/NH
3 followed by protonation of the CH
3Te−
Na+
(sodium methanetellurolate) with sulfuric acid.[1] Few publications describe this compound as a consequence of its instability and paucity of applications.
According to IR spectroscopy, νTe-H = 1995 cm−1. For the lighter homologues, νE-H = 2342 (E = Se), 2606 (E = S), and 3710 cm−1 (E = O) for methaneselenol, methanethiol, and methanol.[1]
References
- ↑ 1.0 1.1 Hamada, K.; Morishita, H. (1977). "The Synthesis and the Raman and Infrared Spectra of Methanetellurol". Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry 7 (4): 355–366. doi:10.1080/00945717708069709.
 |

