Chemistry:Monosodium tartrate
From HandWiki
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium hydrogen tartrate
| |||
| Systematic IUPAC name
Sodium 3-carboxy-2,3-dihydroxypropionate | |||
| Other names
Sodium bitartrate; E335
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
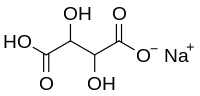
| C4H5NaO6 | |||
| Molar mass | 172.07 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
Monosodium tartrate or sodium bitartrate is a sodium acid salt of tartaric acid. As a food additive it is used as an acidity regulator and is known by the E number E335. As an analytical reagent, it can be used in a test for ammonium cation which gives a white precipitate.[1][2][3]
See also
- Sodium tartrate, the disodium salt of tartaric acid
References
- ↑ Younes, Maged; Aquilina, Gabriele; Castle, Laurence; Engel, Karl-Heinz; Fowler, Paul; Frutos Fernandez, Maria Jose; Fürst, Peter; Gürtler, Rainer et al. (2020-03-11). "Re-evaluation of l(+)-tartaric acid (E334), sodium tartrates (E335), potassium tartrates (E336), potassium sodium tartrate (E337) and calcium tartrate (E354) as food additives". EFSA Journal 18 (3): e06030. doi:10.2903/j.efsa.2020.6030. ISSN 1831-4732. PMID 32874248.
- ↑ Rusyniak, Daniel E.; Durant, Pamela J.; Mowry, James B.; Johnson, Jo A.; Sanftleben, Jayne A.; Smith, Joanne M. (2012-08-28). "Life-threatening hyperkalemia from cream of tartar ingestion". Journal of Medical Toxicology 9 (1): 79–81. doi:10.1007/s13181-012-0255-x. ISSN 1937-6995. PMID 22926733.
- ↑ "Potassium Acid Tartrate Handling/Processing". Technical Evaluation Report 15 (16): 11. January 11, 2017. https://www.ams.usda.gov/sites/default/files/media/Potassium%20Acid%20Tartrate%20TR%20Final%2001%2011%2017.pdf.
 |